Figure 2.
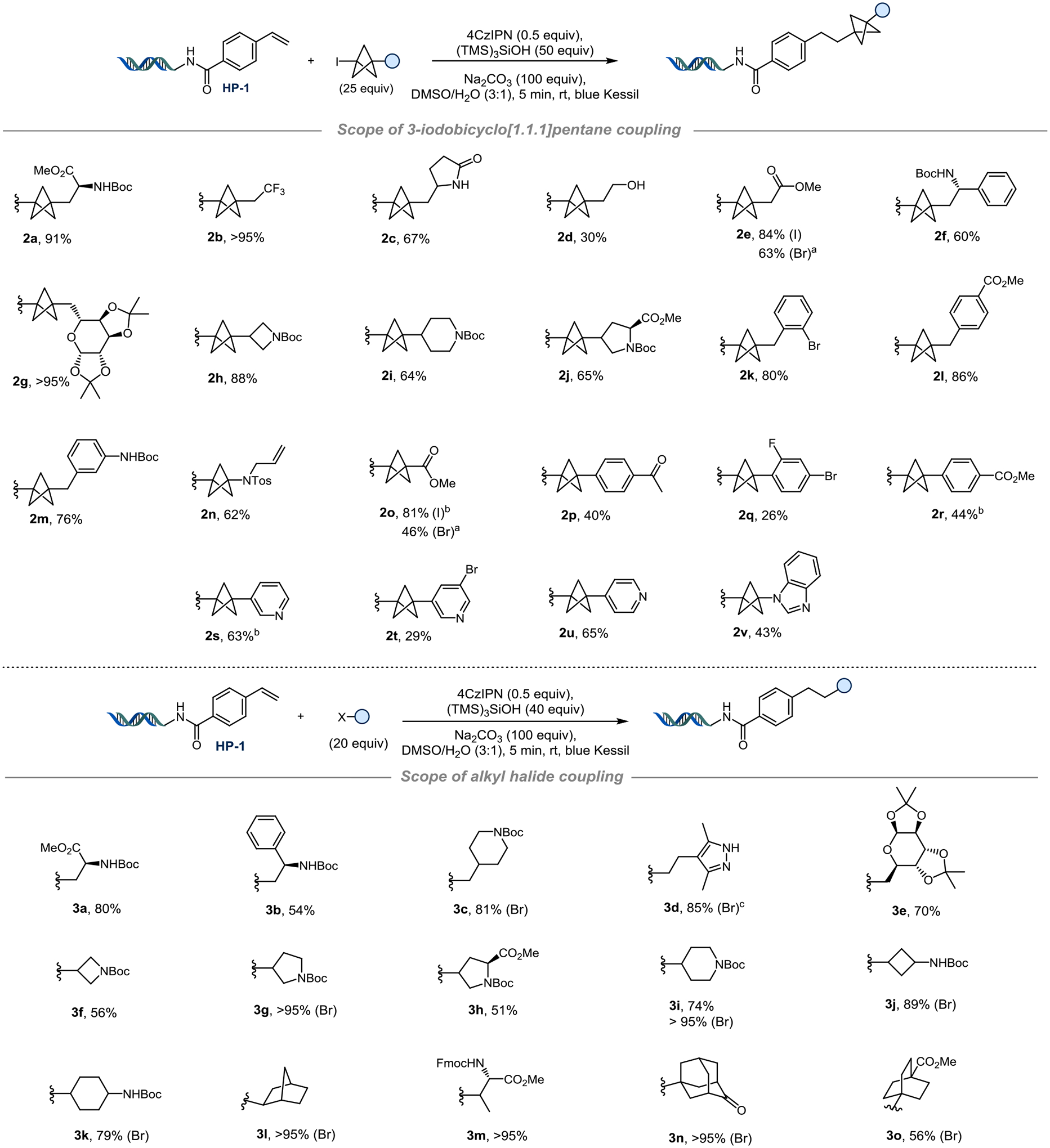
Top. Scope of radical coupling with 3-iodobicyclo[1.1.1]pentanes. The reaction was performed on 10 nmol scale for HP-1 (2 mM in H2O, 1.0 equiv), 4CzIPN (1 mM in DMSO, 0.5 equiv), BCP-I (25 mM in DMSO, 25 equiv), (TMS)3SiOH (50 mM in DMSO, 50 equiv), Na2CO3 (400 mM in H2O, 100 equiv), rt, 5 min, blue Kessil. a) 50 equiv of BCP-I (50 mM in DMSO) and 100 equiv of (TMS)3SiOH (100 mM in DMSO). b) 20 equiv of BCP-I (20 mM in DMSO) and 40 equiv of (TMS)3SiOH (40 mM in DMSO). Bottom. Evaluation of primary, secondary, and tertiary halides. Yields are indicated for alkyl iodides unless otherwise stated. HP-1 (2 mM in H2O, 10 nmol, 1 equiv), 4CzIPN (1 mM in DMSO, 0.5 equiv), alkyl halide (20 mM in DMSO, 20 equiv), (TMS)3SiOH (40 mM in DMSO, 40 equiv), Na2CO3 (400 mM in H2O, 100 equiv), rt, 5 min, blue Kessil. c) 40 equiv of alkyl-Br (40 mM in DMSO) and 40 equiv of (TMS)3SiOH (40 mM in DMSO)
