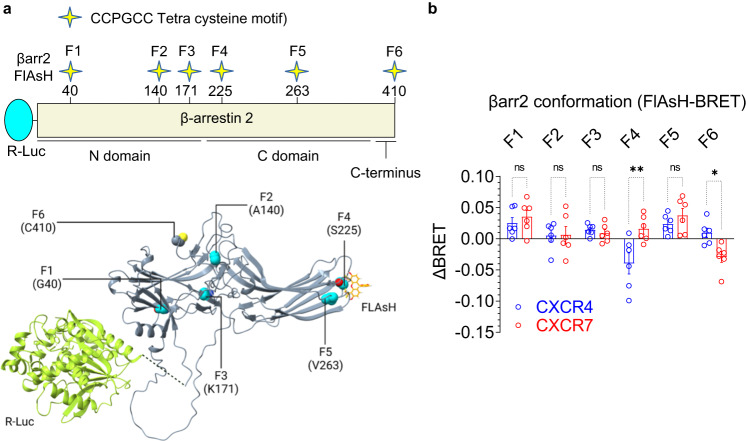
Fig. 6. Conformational changes in βarr2 upon interaction with CXCR4 vs. CXCR7.
a Schematic representation of intramolecular BRET-based sensors of βarr2 conformation where the N-terminus of βarr2 harbors R-Luc (Renilla luciferase) as the BRET donor, and the FlAsH motif are engineered at indicated positions in βarr2 as BRET acceptor. The structural representation in the lower panel is designed based on alpha fold generated model of βarr2. b CXCL12-induced BRET signal measured in HEK-293 cells expressing the indicated receptor and sensor constructs (mean ± SEM), n = 6 independent experiments; the difference of the net-BRET ratio between the stimulated and unstimulated condition were plotted, Two-way ANOVA, Sidak’s multiple comparison test. The exact p-values are as follows: F1:CXCR4 vs. F1:CXCR7 (p = 0.9746), F2:CXCR4 vs. F2:CXCR7 (p > 0.9999), F3:CXCR4 vs. F3:CXCR7 (p = 0.9991), F4:CXCR4 vs. F4:CXCR7 (0.0010), F5:CXCR4 vs. F5:CXCR7 (p = 0.9052), F6:CXCR4 vs. F6:CXCR7 (p = 0.0420) (*p < 0.05, **p < 0.01, ns non-significant). Source data are provided as a source data file.