Fig. 1.
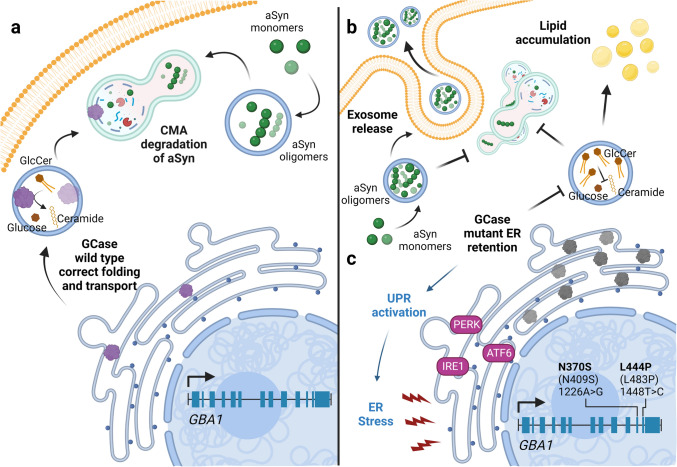
Putative loss- and gain-of-function effects of GCase mutations. a GBA1 encodes for GCase. Wild type enzyme (purple protein) is correctly folded and can be transported to the lysosomes (blue complete circles) where it hydrolyzes glucosylceramide (GlcCer) into glucose and ceramide. This contributes to the correct function of the autophagic system which, through the Chaperone Mediated Autophagy (CMA) pathway, is able to degrade proteins and prevent their accumulation, for example aSyn. b In the loss-of-function hypothesis due to GBA1 mutations, unfolded GCase cannot be transported to the lysosome, sphingolipid metabolism is compromised and GlcCer is accumulated. This also impairs the formation of autophagolysosomes, promoting the accumulation of aSyn oligomeric forms inside the cell. To reduce aSyn burden, changes in exosomal-mediated release of aSyn may take place. c In the gain-of-function hypothesis, the retention of mutant GCase in the ER activates the UPR response proteins (PERK, IRE1 and ATF6), generating ER stress which may, in turn, alter lipidostasis