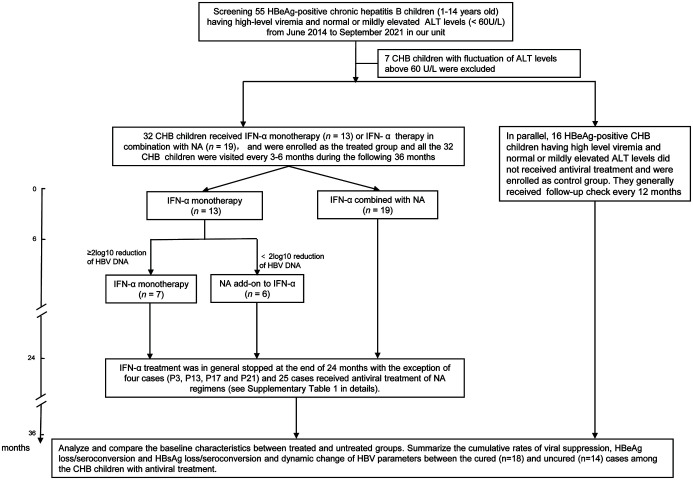
Fig. 1. Flow diagram of antiviral course of treatment in children with CHB having high-level viremia and normal or mildly elevated serum ALT levels.
Fifty-five children 1–14 years of age) were screened, and 48 patients who met the inclusion/exclusion criteria were included in this study. Thirty-two patients received antiviral treatment consecutively and the remaining 16 made up the untreated control group. In the treated group, the duration of IFN-α monotherapy among the cured children depended on the time point when serum HBsAg loss and anti-HBs positivity occurred. The study data, including antiviral regimens, are shown in detail in Table 1. We analyzed the rate of functional cure in children with CHB in the immune-tolerant phase at 36 months. ALT, alanine aminotransferase; CHB, chronic hepatitis B; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; IFN-α: interferon-α; NA, nucleoside analog.