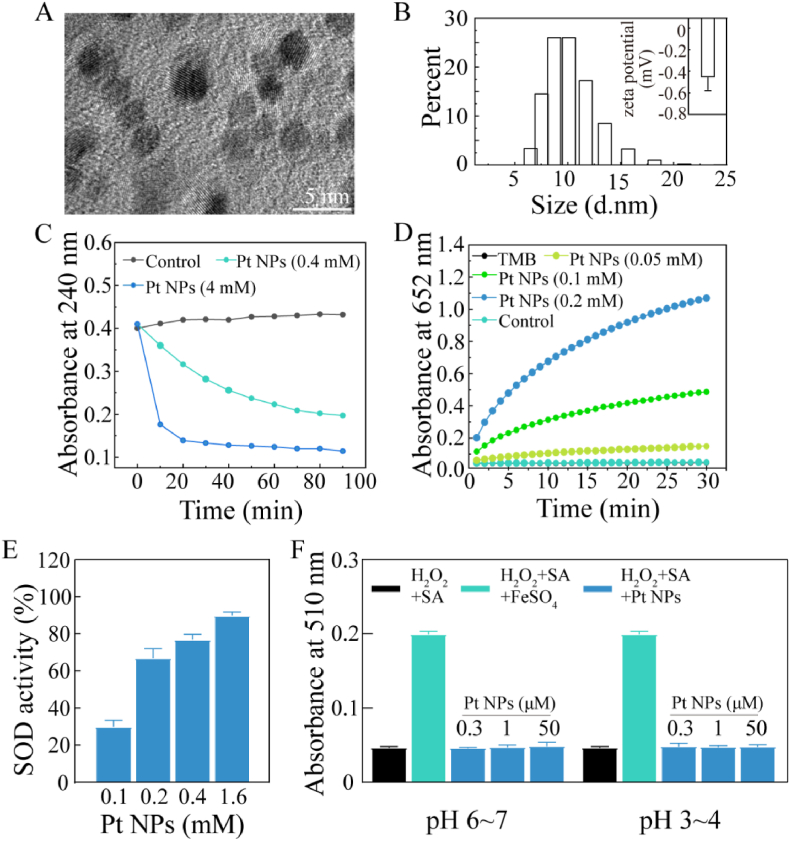
Fig. 1.
Physical and chemical characterizations of Pt NPs. (A) TEM image of Pt NPs. (B) The size distribution of Pt NPs. The insert was zeta potential of Pt NPs. (C) CAT-like activity: time-dependent UV–visible absorption at 240 nm of H2O2 solution after incubating with Pt NPs. H2O2 alone was considered as control. (D) POD-like activity: time-dependent UV–visible absorption at 652 nm of the solution after incubating with Pt NPs. TMB alone (black) and TMB mixing with H2O2 (cyan) as control. (E) SOD-like activity: concentration-dependent SOD activity of Pt NPs at increased concentrations. (F) No Fenton-reaction-like activity of Pt NPs: H2O2 mixing with SA, FeSO4 or without FeSO4 was used as control, respectively. The mixture of H2O2, SA and various concentrations of Pt NPs (blue) was used for testing. The Pt NPs were pre-incubation wiht PBS (pH 6–7) or acetate buffer (pH 3–4).