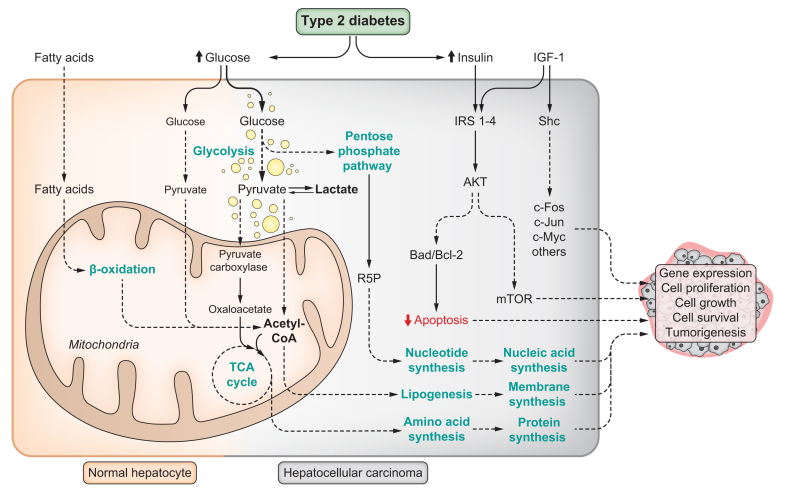
Fig. 3.
Hepatocyte transformation in NAFLD/T2D.
Normal hepatocytes prefer β-oxidation of fatty acids as a source of energy, as well as relatively low glucose uptake and oxidation. This is especially true during fasting conditions and this preference is influenced by hepatic zonation. Transformed hepatocytes exhibit high glucose uptake and rely on aerobic glycolysis as a source of energy. Pyruvate is preferentially converted into lactate, instead of being oxidised in the mitochondria. High fat intake rewires hepatocyte energy metabolism to favour glucose uptake and its utilisation as a source of energy through aerobic glycolysis and lactate production. Glucose can be used as a carbon source for biosynthetic reactions in rapidly growing tissues, as well as in cell signalling and maintenance of the redox state. High glucose uptake also sustains the substrate requirement for the pentose phosphate pathway. This is important for ribulose-5-phosphate synthesis, which is required for nucleotide biosynthesis and nucleic acid replication. Pyruvate carboxylase is induced by high-fat intake, favouring the entrance of pyruvate into the TCA cycle as oxaloacetate to maintain anaplerotic reactions required for amino acid biosynthesis. Acetyl-CoA is converted into fatty acids through lipogenesis. The pool of intracellular fatty acids (from lipogenesis and extrahepatic tissues) is used for phospholipid biosynthesis to build biological membranes. Hyperinsulinemia associated with T2D, in combination with the action of the hepatokine IGF-1, can lead to dysfunctional signalling pathways involved in cell survival, apoptosis, and stress responses. Together, these cellular and metabolic changes can represent advantages for cancer cells, allowing them to sustain rapid growth and proliferation. Bad, Bcl-2-associated agonist of cell death; Bcl-2, B-cell lymphoma 2; HCC, hepatocellular carcinoma; IGF-1, insulin-like growth factor 1; IRS1-4, insulin receptor substrate 1-4; mTOR, mammalian target of rapamycin; NAFLD, non-alcoholic fatty liver disease; Shc, Src homology 2 domain-containing transforming protein; T2D, type 2 diabetes; TCA, tricarboxylic acid.