Abstract
A cancer diagnosis is life altering and frequently associated with both acute and long-lasting psychosocial and behavioral distress for patients. The impact of a diffuse glioma diagnosis on mental health is an important aspect of the patient experience with their disease. This needs to be understood by neurosurgeons so these concerns can be appropriately addressed in a timely fashion and integrated into the multidisciplinary care of neuro-oncology patients. The relatively grave prognosis associated with diffuse gliomas, the morbidity associated with treatment, and the constant threat of developing a new neurological deficit all can negatively affect a patient’s mental ability to cope and ultimately manifest in mental health disorders like anxiety and depression. The objective of this review was to describe the variety of behavioral health disorders patients may experience following of a glioma diagnosis and discuss possible treatment options. Given the strong correlation between quality of life and patient mental well being, there is a considerable need for early recognition and treatment of these behavioral health disorders to optimize everyday functioning for patients.
Keywords: Glioma, Primary Brain Tumor, Mental Health, Psychosocial, Quality of Life, Neurobehavioral
Introduction
Diffuse gliomas represent the most common intrinsic brain tumor in adults and, despite treatment advances, patients must navigate life with the knowledge of this somber diagnosis and the constant threat of tumor recurrence. The treatment of these tumors relies on a multidisciplinary care team comprising neurosurgeons, neuro-oncologists, and radiation oncologists and prioritizes prolonging a high quality of life (QOL) and minimizing neurological deficits. Although the presence of this serious diagnosis frequently has a severe behavioral and psychological impact on the patient, professional psychological services may not be offered. During surgery, adjuvant therapy, disease surveillance, or times of disease progression, patients may experience psychiatric or behavioral symptoms that impact their social interactions, QOL, mood, and even their clinical decision-making. These changes in personality, mood, and behavior can be challenging to recognize and treat, as well as substantially impact both the patient and their loved ones who become their informal caregivers. In addition, their QOL can be negatively impacted by commonly utilized medications such as, corticosteroids and anti-epileptics, that often have behavioral side-effects. Additionally, the stress and emotional toll from the diagnosis can lead to feelings of sadness and interfere with everyday functioning.1 In this systematic review, we describe the relevance of mental health disorders in patients with intra-axial brain tumors, the role for interventions that address both long-standing and new mental health conditions, and the importance of recognizing this aspect of patient care for neurosurgeons.
Methods
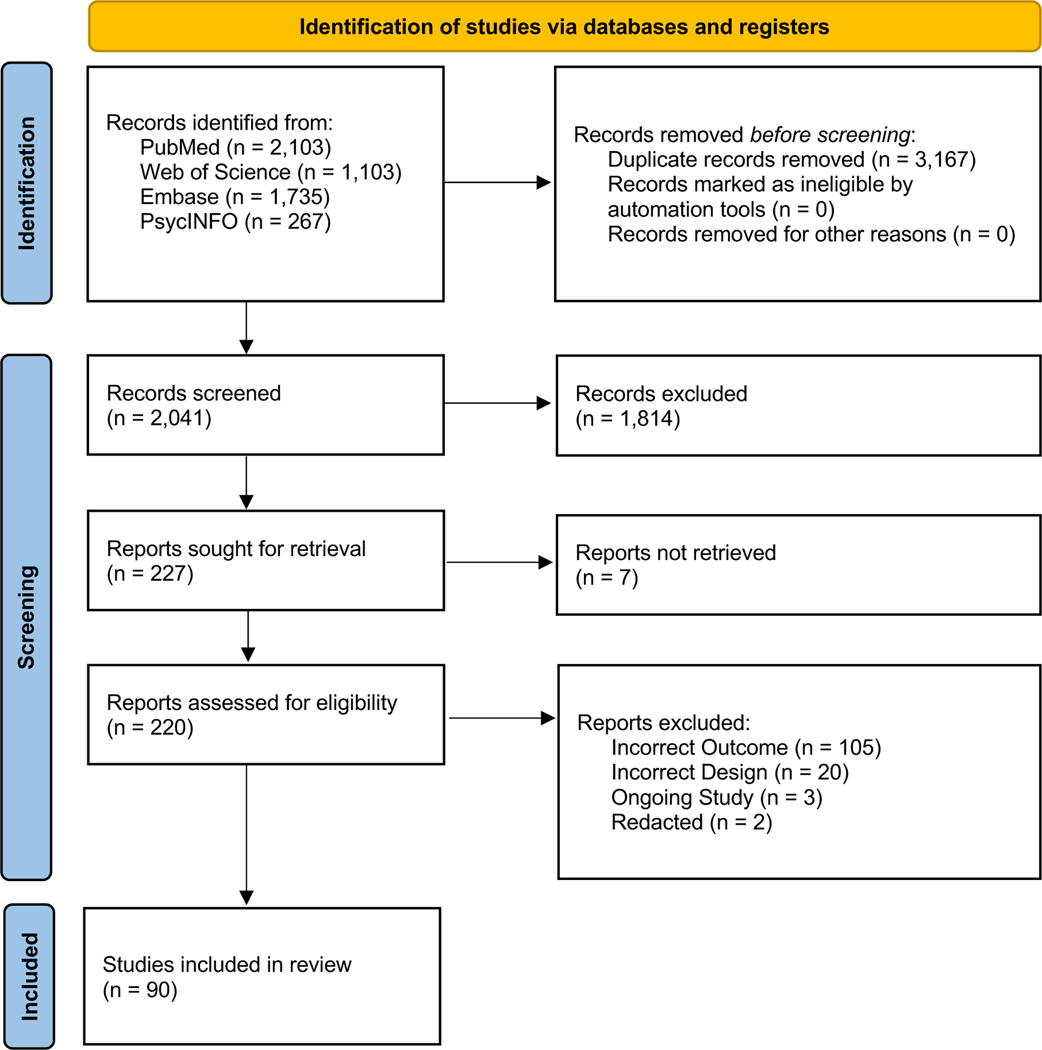
A literature search, guided by the Preferred Reporting Items for Systematic Reviews (PRISMA) (Figure 1), was performed of the following electronic databases through July 1st, 2022: PubMed, Web of Science, Embase, and PsycINFO. Search terms included MeSH terms and relevant word combinations (Online Appendix 1). Covidence systematic review software was used for duplicate detection and screening. Two reviewers conducted the title and abstract screening, full-text review, and extraction. Conflicts were handled through discussion or, if necessary, by consulting another author. Following full-text review, studies were categorized qualitatively according to the following subtopics: generic discussion, anxiety, depression, distress, stress, pharmacology, interventions, and caregivers.
Figure 1.
Preferred Reporting Items for Systematic Reviews (PRISMA) Flow Diagram of Literature Search Performed.
Studies reported in English were included if the population included those with a Glioma diagnosis and the primary aim was the assessment of mental health related outcomes (i.e., depression, anxiety, mood, etc.). Exclusion criteria included animal studies, studies with a heterogenous patient population without specifically reporting outcomes for Glioma patients, and those that indirectly reported mental health related outcomes.
Results
A total of 5,208 studies were identified and uploaded to Covidence. After the removal of duplicates (n = 3167), 2,041 title and abstracts were screened. Of the 227 studies sought for full-text review, 220 full-texts were available for review. A total of 145 additional studies were excluded due to incorrect outcomes (n = 120), incorrect design (n = 20), ongoing study (n = 3), or the study was redacted (n = 2). Our literature search culminated in 75 articles which continued to extraction.
Domains of Emotional and Social Dysfunction in Brain Tumor Patients
Patients with diffuse gliomas may experience dysfunction in multiple mental health domains that neurosurgeons should be familiar with (see Table 1 for summary). In fact, although physical deficits dominate health-related QOL scores for patients early in their disease course, psychological, emotional, and cognitive dysfunction become the most common complaints reported by longer-term survivors.2 As such, for patients with low-grade gliomas (LGGs) specifically, with longer life expectancies than high-grade gliomas (HGGs), it is even more imperative for neurosurgeons and neuro-oncologists to recognize the presence of mental health disorders (MHDs) that could negatively impact quality-of-life. In fact, a recent report utilized the IBM Watson Health MarketScan Database explored the incidence, prevalence, and risk factors of MHDs for LGG patients. Overall, 60.9% of patients had at least MHD before or after their diagnosis. In patients without a pre-existing MHD, nearly 17% received a first time diagnosis within one year of their LGG diagnosis.3 Importantly for surgeons, they reported biopsy or surgical resection, compared to no surgical treatment, female gender, seizures, and age, between 34 and 45 years, as unadjusted risk factors for MHDs. Perhaps more applicable to the clinician, variability may exist according to treatment stage, tumor grade, and assessment timing. Studies have reported a greater prevalence of MHDs in HGG patients but only at 6 months following surgery.4 In LGG patients, 3 months post-operative, MHDs were more prevalent in those receiving radiation compared to no therapy at all.4 On the other hand, in a multivariate regression analysis of mood disturbances (MDs) in a cohort of 186 glioma patients, risk factors depended on whether they were new or recurrent patients. In the former, steroids and marital status were protective factors while anticonvulsant and steroid use in low-income patients with recurrent tumors were significant risk factors for MDs.5 From these two studies alone, it is evident that there is variability in factors associated with MHDs overall, and a large interplay between socioeconomic and clinical factors likely exist.
Table 1. Summary of Common Behavioral Health Disorders in Brain Tumor Patients.
Although there is a wide range of reported incidence for mental health disorders in glioma patients, and the evidence supporting treatment of these conditions in this patient population is limited, the table below highlights the impact these conditions have on patients and risk factors for their development.
| Behavioral Health Disorder | Incidence | Risk Factors | Impact on Patient Care | Treatment Options | References |
|---|---|---|---|---|---|
|
| |||||
| Anxiety | 35–50% | • Prior anxiety disorder • Female gender • Prior psychiatric disorder • Lower WHO grade tumor |
• Longer hospitalization • More health care utilization • Increased depressive symptoms • Worse QOL |
• Pharmacotherapy • Psychotherapy |
73, 74 |
| Depression | 15–27% | • Female gender • Marital status • Comorbid medical conditions • Higher WHO grade tumor |
• Shorter survival • Impaired QOL • Memory impairment • Neurologic complications • Non-routine discharge |
• Antidepressants • Psychotherapy |
9, 20, 75 |
| Distress/Despair | 74% | • Higher WHO grade tumor | • Impaired QOL | • Psychotherapy | 37 |
| Stress/PTSD | >60% | • Longer hospitalizations • Complications |
• Development of post traumatic disorder | • Education on stress reduction techniques • Psychotherapy |
45 |
Anxiety
Anxiety, which is already common amongst the general population, is nearly universal amongst patients scheduled for neurosurgery, with reported preoperative rates of anxiety and severe anxiety in over 80% and 55% of patients, respectively.6,7 Given the potential risks of surgical intervention for diffuse glioma, this high prevalence is not particularly surprising.8 However, many do not receive psychological or pharmacological treatment for their anxiety. For example, one study reported that while 48% of neuro-oncology patients had generalized anxiety disorder (GAD), only 31% were receiving medical treatment for their GAD, suggesting anxiety symptoms in this population may be undertreated.9 Importantly, although there was no association with survival, preoperative anxiety was associated with longer hospitalizations, a higher need for information from health care providers, increased depressive symptoms, more physical disability, worse QOL 6, and post-operative pain10. As such, there remains a large potential value of treating these patients for anxiety, alone.6 Other important risk factors for anxiety may be time and grade dependent. Studies reporting risk factors at certain time points are scarce and lack clinical applicability however, they implore an important take away such that, the risks associated with Temozolomide (TMZ) and steroid use is stable across all patient groups and time intervals, which may be more important for the clinician.11 Although relatively few studies have investigated if awake craniotomies induce more anxiety than asleep procedures, two studies found no difference in pre-operative rates.12,13 Following surgery, glioma patients frequently continue to experience fear and anxiety that contributes to an overall lower QOL and may warrant either behavioral counseling or medical management.14,15
Additionally, many brain tumor patients may suffer from ‘scanxiety,’ a phenomenon that occurs when patients undergo a routine scan and follow-up with their care team. Surveillance scans are a critical aspect of patient management but there is varied guidance on the utility of regular scans when juxtaposed with the potential for anxiety with each scan. This phenomenon is not unique to brain tumor patients, as over 80% of lung cancer patients have anxiety regarding scans.16 While patient anxiety decreases with a stable scan result, there is a significant positive relationship between the scan-to-discussion interval and severity of symptoms and long-lasting distress. These findings underscore the need for long-term and consistent assessment and treatment for anxiety related symptoms.
Depression
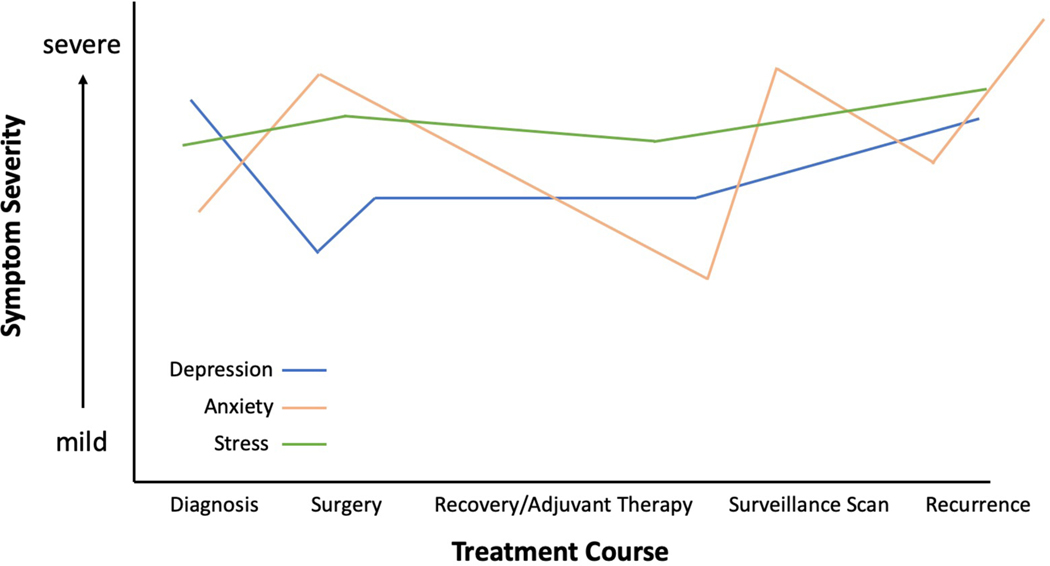
The prevalence of depressive symptoms in primary brain tumor(PBT) patients during the course of their disease varies widely between studies, ranging from 5% to 44%17–19, and is may be time-dependent (Figure 2)20. Given the relatively high rates of depression in glioma patients, some authors suggest screening one month after diagnosis to allow for a common and expected initial period of sadness.21 Depression has been shown to predict worse survival outcomes in some studies even after controlling for functional status, tumor grade, and treatment17,18,20,22. While this relationship was true for both pre- and post-operative diagnosis, pre-operative depression appeared to be more significantly related to outcomes in a recent meta-analysis22. Data from the Glioma Outcomes Project found a significant gap between high-grade glioma patients’ self-reported rates and those recognized by physicians, with over 90% of patients reporting symptoms but only 15% of physician recognition. This study found an increase in depressive symptoms in the 6-month post-operative period and revealed an association with survival outcomes,8,23 similar to other studies. Notably, depressive symptoms appear to be one of the most important predictors of a lower post-operative QOL24,25 and are associated with higher rates of memory impairment, neurologic complications, and nonroutine discharges.26,27
Figure 2. Schematic Demonstrating Variability in Symptom Severity Over Time in Brain Tumor Patients.
Certain times during the patient’s treatment course, such as at the time of diagnosis, surgery, surveillance scanning, or tumor recurrence, maybe associated with elevated levels of anxiety or depression. Stress-levels have been reported to remain elevated throughout a patient’s entire time with a disease.
In addition to recognizing the prevalence and effect of depression on outcomes in these patients, practitioners should be aware of some of the risk factors, as well. Intuitively, patients with anteriorly located28 or higher-grade tumors29 have increased levels of depression, which is similar to the increased anxiety seen in right-sided tumors30. Wellisch et al. reported that a frontal region tumor, sadness, lack of motivation, and a positive psychiatric family history were all risk factors for a Diagnostic and Statistical Manual of Mental Disorders (DSM) diagnosis of major depressive disorder (MDD) on multivariate analysis.31 Interestingly, compared to their counterparts, patients with frontal lobe tumors are more amenable to relief of these psychiatric symptoms following resection.28 Regarding treatment, while increased rates of depression have been reported in patients undergoing a second operation32, multivariate analyses have shown that only pre-operative functional status is associated with depression while controlling for employment status, coping strategies, insurance status, treatment stage, steroid use, surgical intervention, extra ventricular drain placement, number of admissions, number of operations, histology, recurrence, and structures involved.33 Similar studies, in line with these analyses have widely reported the lack of association between treatment stage, timing of treatment, and various demographic datapoints, suggesting a largely global prevalence of depression in these patients.13,34–36 On the other hand, a study evaluating hope found that patients with recurrent tumors experienced lower levels of interconnectedness and overall hope on the Herth Hope Index (HHI) as well as, significantly greater mood disturbances.37
Although depression is common in this population, treatment is inconsistent. Studies have reported that only 36% to 63% of patients, identified as having symptoms of depression, were prescribed an antidepressant or other psychotropic medication.25 Similarly, in 788 patients evaluated in the Glioma Outcomes Project, only 6.7% received antidepressants in the perioperative period.38 This possible under-prescription may be a result of poor physician recognition of patient-reported symptoms39, or perhaps due to physicians ascribing these symptoms to tumor-related structural sequalae. Interestingly, type of and number of years in practice have a significant impact.40 Accordingly, neurosurgeons at research institutions were less likely to prescribe antidepressants while, general and brain tumor neurosurgeons were more likely to do so.40 In parallel with these prescribing rates, current trends for screening41, despite resource availability, and referral to mental health services42 are equally concerning. On a promising note, a recent nationwide matched LGG study reported increases in the use of antidepressants within recent years.43 Nonetheless, given the significant impact of depression on survival and QOL, interventions to target these symptoms could provide substantial benefit.
Distress/Despair
Distress, defined by the National Comprehensive Cancer Network (NCCN) as “a multidetermined unpleasant emotional experience of a psychological, social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms and its treatment,” is extremely common and long-lasting44 among PBT patients, with up to 74% reporting feelings of distress at some point in the course of their disease45. As suspected, very few of these patients go on to receive psychiatric care.46 Distress and Despair are closely associated with depression and additional physical and emotional impairments. Fatigue, memory and concentration impairment, nervousness, and worry, are the most commonly reported symptoms contributing to feelings of distress.47 PBT patients, in particular, have a high prevalence of death-related distress, especially death anxiety, which contributes significantly to overall distress.14 Multiple studies have reported that newly diagnosed patients suffer from higher levels of distress when compared to patients greater than a year out from diagnosis, highlighting the importance of early recognition and intervention.39,47 Not surprisingly, distress scores as measured on the NCCN-Distress Thermometer show a significant inverse relationship with QOL, including lower social and emotional well-being.48 These findings, taken together, suggest that the early phase post-diagnosis is associated with a high risk of distress and possible depression for patients with a PBT. Consequently, early interventions to target MHDs could be beneficial in reducing levels of distress in such patients.
Stress
Greater than 60% of PBT patients, regardless of tumor grade, report elevated levels of stress, which is defined as the psychological or physical response that occurs when adapting to changing conditions.49,50 While initial diagnosis can understandably invoke high levels of stress, they appear to persist in longer term survivors.50,51 Of particular concern are the implications of stress severity, as rates of Post-Traumatic Stress Disorder (PTSD) have been reported to be as high as 16% in LGG patients only 3-months following surgery.52 Overall however, PTSD is understudied in patients with PBTs53 although, it is a well-documented phenomenon amongst other cancer patients. In one cross-sectional study of inpatients with both malignant and benign brain tumors, the former experienced higher levels of clinically relevant post-traumatic stress symptoms (PTSS), which could be explained by the provocation of fears of death due to the certainty of tumor progression and poor survival rates.54 Additionally, stress from the diagnosis may also affect the caregiver. In one study of pediatric brain tumor survivors, roughly a third of both the survivors and their parents reported severe levels of PTSS.55 In the patients, longer duration of hospitalization was associated with increased PTSS, while a higher number of tumor recurrences was associated with PTSS in their parents.55 Future work studying the risk factors for the development of PTSD and the potential benefit of early incorporation of behavioral health counseling services in the management of brain tumor patients are urgently needed. While targeted treatments such as, Eye Movement Desensitizing Reprocessing (EMDR)56 and reminiscence57 therapy have shown promise as non-pharmacological options, additional evidence is needed regarding feasibility and safety.
Corticosteroids, Anti-Epileptics, and Behavioral Disturbances
Many of the medications used in the management of glioma patients can have behavioral side effects that are important for neurosurgeons to be aware of. For example, Corticosteroids, which are commonly prescribed to manage vasogenic cerebral edema, can induce symptoms of psychosis and insomnia.58 As a result of an altered sleep-wake cycle and hyperarousal, corticosteroids may induce delirium and exacerbate other behavioral disorders.51 One retrospective study of 340 recurrent glioma patients found the incidence of insomnia was 52% in patients taking corticosteroids, the severity of which appeared to correlate with dose.59 As a result, interventions to mitigate the disturbance of sleep including sedative-hypnotics and other sleep aids including melatonin and reinforcement of sleep hygiene including avoidance of caffeine late in the day may help minimize these side effects and improve QOL while taking steroids. Neurosurgeons should be mindful of these side-effects and attempt to wean steroids as quickly as possible when appropriate.
Levetiracetam, which has become the first-line anti-epileptic medication (AED) for brain tumor patients, due to its limited interaction with other medications, has also been implicated in the development of behavioral side-effects60, a risk that may be heightened in the presence of an anteriorly located tumor61. The most commonly reported side effects in retrospective studies include depression, fatigue, and irritability. Although the severity of these side-effects rarely warrant discontinuation62, in rare instances, patients have developed psychosis or visual hallucinations indicating cessation63. Interestingly, in glioma patients, a recent meta-analysis found no association between AED use and concurrent depression, anxiety, or subjective cognitive impairment.64 Nevertheless, neurosurgeons should be aware of these potential drugrelated side effects particularly in patients with a history of MHD and/or symptoms after starting this medication post-operatively.
Can Behavioral Health and Pharmacological Interventions Improve Mood and Patient Quality of Life?
Given the high prevalence of psychiatric, emotional, and neurobehavioral symptoms among patients with PBTs, coupled with their significant impact on QOL, there is a high level of interest in developing targeted interventions. Literature exploring this topic, particularly in PBT patients, is limited. A recent systematic review reported ten studies with promising results.65 One randomized controlled trial reported that a home-based psychosocial intervention for brain tumor patients enhanced well-being and QOL.66 In this study, patients who received 10 onehour weekly sessions on psychoeducation, cognitive rehabilitation, psychotherapy, and couple and family support had significantly lower levels of depression and higher levels of existential and functional well-being. These findings support a home-based intervention as a beneficial adjunct to their oncologic treatment regimen.66 Another study of brain tumor patients undergoing post-operative radiotherapy found that for patients identified as being distressed at baseline, an intensive schedule of psychological therapy significantly improved their distress levels, mood, and overall QOL over time.67 In a similar cohort, Eisenhut et al. studied the effects of different exercise programs in patients with HGGs and reported that endurance training was superior to strength training in improving psychiatric symptoms.68 Interestingly, their active control group, which simply met twice weekly to share their experiences also outperformed the strength training group, which suggests that a connection with other cancer patients should be of priority, as well.
Given the success of these home-based interventions, there is considerable interest to develop interventions that can be delivered remotely to ease the burden of participation and improve access to care. This is especially relevant for brain tumor patients, whose neurological symptoms can make travelling to in-person services challenging. Studies have demonstrated that telephone-administered psychotherapy is effective in reducing depressive symptoms in non-tumor patients69, and one small-scale study aimed at investigating the feasibility and utility of telephone-based psychotherapy for patients with a brain tumor also demonstrated promising results. The study enrolled four patients who received 10 telephone-based therapy sessions followed by a booster session four weeks after completing the initial sessions. Sessions covered topics such as mindfulness and relaxation techniques, couple and family support, and existential and end-of-life discussions. Although this study was small, all four participants reported improved QOL post-treatment as well as a strong therapeutic alliance with their providers. Compliance to behavioral care is often poor but all four participants successfully completed the telephone-based therapy.70 Taken together, these studies suggest that behavioral health interventions, including those delivered remotely via telephone or Telehealth in the post COVID-19 era, can be effective in improving QOL for patients with a PBT.
Pharmacological management of anxiety and depression has been surprisingly understudied in cancer patients broadly and certainly in the glioma patients. In fact, there is no randomized trial investigating antidepressants in glioma patients and Cochrane reviews have reported no highquality studies71. Retrospective studies suggest that selective serotonin reuptake inhibitors (SSRIs) are safe to use and do not appear to negatively influence complications or survival72 and, although widely used in the treatment of depression and anxiety, they do not appear to improve overall survival in glioblastoma multiforme (GBM) patients73. Nevertheless, in a recent meta-analysis investigating the treatment of depression in cancer patients, older than 65 years of age, of various etiologies, there was a general trend towards improved behavioral health and mood in patients treated with SSRIs and SNRIs.74 On the other hand, in patients with advanced cancer and a high-risk of depression (The Hospital Anxiety/Depression Scale [HADS] score ≥ 15), Pu et al. reported rates of mood improvement exceeding 50% within less than 3 weeks as well as, improved treatment tolerance and associated side effects.75 Taken together, these reports may suggest not only that these drugs may be of use in brain tumor patients but also, that future studies should tailor their inclusion criteria towards those with clinically significant symptoms for optimal applicability. Although outside the scope of this review, there is also some preclinical evidence that SSRIs have a direct anti-cancer effect by inhibiting a lipid metabolism enzyme, SMPD1, which has motivated a large investment by the National Brain Tumor Society (NBTS) to explore fluoxetine (Prozac) treatment for GBM76.
Another active area of interest for the treatment of psychiatric and MHDs in brain tumor patients is the potential role of medicinal cannabinoids.77,78 Although preclinical animal studies are promising, and there is evidence that cannabinoids can improve cancer-related pain and stimulate appetite, patients in early-phase trials have reported some side-effects associated with their use. 79 Additional studies are ongoing that evaluate whether medicinal cannabis, prescribed in conjunction with cognitive behavioral therapy and other psychopharmacological drugs, can aid in treating both MHDs and treatment-related insomnia in cancer patients.
Mental Health Struggles for Caregivers
A brain tumor diagnosis impacts more than just the patient’s life. Due to the nature of these tumors and the intensive treatments they require, patients frequently rely on loved ones and friends to serve as caregivers. The role is associated with complex feelings that can range from rewarding to stressful and mentally exhausting.80,81 Caregivers even report feeling a sense of total responsibility for all aspects of patient care. As the patient is their primary focus, caregivers can find it difficult to obtain emotional support for themselves and face a lack of education and information on how to care for the patient leading to further feelings of anxiety.81 And although caregivers face many challenges, ranging from the physical demands of caring for the patient to the financial burden of taking time away from their own employment, they often describe the neuropsychiatric symptoms the patient exhibits as the most challenging to deal with and as taking the largest toll on their own mental health.81,82 Greater patient reported neuropsychiatric symptoms has been associated with a higher level of caregiver depression, whereas patient tumor grade, cognitive status and independent activities of daily living (IADL) status do not predict caregiver distress80,82. Some additional risk factors for distress in care givers of brain tumor patients are female gender and rural location.83 Not surprisingly, caregivers of patients with brain tumors experience a decrease in QOL and higher levels of anxiety and depression symptoms compared to the general population80. Moreover, these symptoms are long lasting with studies reporting persistence over a 9-month period.84 Longitudinal studies have demonstrated that these symptoms may necessitate treatment, as well. Compared to their counterparts, partners of glioma patients had a more than 4-fold risk of receiving a psychiatric prescription within the first year of diagnosis and that, within the first two years, nearly 30% of them had a first-time prescription.85
Incredibly, when surveyed, caregivers report a higher number of unmet care needs than patients. Two of the most reported unmet needs for caregivers were assistance in reducing stress in the life of patients with a brain tumor and help in managing difficult aspects in the behavior of these patients.86 Given these findings, there is a need to provide psychological support not only to the patient but to the caregiver, as well. In fact, a combined treatment program involving dyadic yoga was not only feasible, but also effective at improving psychiatric symptoms in both the caregivers and the patients, who also experienced decreased cancersymptom severity.87 Therefore, while a patient’s care team may struggle to provide medical treatment to their respective caregivers, alternative options that may be benefit both are needed.
Conclusion
Between half to three quarters of brain tumor patients suffer from a behavioral health disorder as a consequence of their diagnosis and corresponding treatment. Neurosurgeons should be aware of the impact a glioma diagnosis has on a patient’s mental well-being. With limited access to behavioral resources, providing services at home to the patient and caregiver through telehealth may be an important intervention in the management of brain tumor patients. By recognizing the stress and emotional toll patients experience as a part of their treatment journey following a glioma diagnosis, glioma surgeons can start the process of addressing these symptoms early in the patient’s treatment course. Principally, neurosurgeons must understand their role in the overall patient experience and be prepared to have an open discussion about these mental health conditions with patients.
Supplementary Material
Acknowledgments:
We do not have any acknowledgements to make.
References
- 1.Lucas MR. Psychosocial implications for the patient with a high-grade glioma. Journal of Neuroscience Nursing. 2010;42(2):104–108. doi: 10.1097/JNN.0b013e3181ce5a34 [DOI] [PubMed] [Google Scholar]
- 2.Frances SM, Velikova G, Klein M, et al. Long-term impact of adult WHO grade II or III gliomas on health-related quality of life: A systematic review. Neurooncol Pract. 2022;9(1):3–17. doi: 10.1093/nop/npab062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhanja D, Ba D, Tuohy K, et al. Association of Low-Grade Glioma Diagnosis and Management Approach with Mental Health Disorders: A MarketScan Analysis 2005–2014. Cancers (Basel). 2022;14(6):1376. doi: 10.3390/cancers14061376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leonetti A, Puglisi G, Rossi M, et al. Factors Influencing Mood Disorders and Health Related Quality of Life in Adults With Glioma: A Longitudinal Study. Front Oncol. 2021;11:662039. doi: 10.3389/fonc.2021.662039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acquaye AA, Vera-Bolanos E, Armstrong TS, Gilbert MR, Lin L. Mood disturbance in glioma patients. J Neurooncol. 2013;113(3):505–512. doi: 10.1007/s11060-013-1143-1 [DOI] [PubMed] [Google Scholar]
- 6.Oteri V, Martinelli A, Crivellaro E, Gigli F. The impact of preoperative anxiety on patients undergoing brain surgery: a systematic review. Neurosurg Rev. 2021;44(6):3047–3057. doi: 10.1007/s10143-021-01498-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perks A, Chakravarti S, Manninen P. Preoperative anxiety in neurosurgical patients. J Neurosurg Anesthesiol. 2009;21(2):127–130. doi: 10.1097/ANA.0b013e31819a6ca3 [DOI] [PubMed] [Google Scholar]
- 8.D’Angelo C, Mirijello A, Leggio L, et al. State and trait anxiety and depression in patients with primary brain tumors before and after surgery: 1-Year longitudinal study. J Neurosurg. 2008;108(2):281–286. doi: 10.3171/JNS/2008/108/2/0281 [DOI] [PubMed] [Google Scholar]
- 9.Arnold SD, Forman LM, Brigidi BD, et al. Evaluation and characterization of generalized anxiety and depression in patients with primary brain tumors. Neuro Oncol. 2008;10(2):171–181. doi: 10.1215/15228517-2007-057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hejrati N, Spieler D, Samuel R, Regli L, Weyerbrock A, Surbeck W. Conscious Experience and Psychological Consequences of Awake Craniotomy. World Neurosurg. 2019;129:e381–e386. doi: 10.1016/j.wneu.2019.05.156 [DOI] [PubMed] [Google Scholar]
- 11.Knudsen-Baas KM, Johannesen TB, Myklebust TÅ, et al. Antiepileptic and psychiatric medication in a nationwide cohort of patients with glioma WHO grade II–IV. J Neurooncol. 2018;140(3):739–748. doi: 10.1007/S11060-018-03007-9/TABLES/4 [DOI] [PubMed] [Google Scholar]
- 12.Santini B, Talacchi A, Squintani G, Casagrande F, Capasso R, Miceli G. Cognitive outcome after awake surgery for tumors in language areas. J Neurooncol. 2012;108(2):319–326. doi: 10.1007/s11060-012-0817-4 [DOI] [PubMed] [Google Scholar]
- 13.Staub-Bartelt F, Radtke O, Hänggi D, Sabel M, Rapp M. Impact of Anticipated Awake Surgery on Psychooncological Distress in Brain Tumor Patients. Front Oncol. 2021;11:795247. doi: 10.3389/fonc.2021.795247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loughan AR, Aslanzadeh FJ, Brechbiel J, et al. Death-related distress in adult primary brain tumor patients. Neurooncol Pract. 2020;7(5):498–506. doi: 10.1093/nop/npaa015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loughan AR, Lanoye A, Aslanzadeh FJ, et al. Fear of Cancer Recurrence and Death Anxiety: Unaddressed Concerns for Adult Neuro-oncology Patients. J Clin Psychol Med Settings. 2021;28(1):16–30. doi: 10.1007/s10880-019-09690-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauml JM, Troxel A, Epperson CN, et al. Scan-associated distress in lung cancer: Quantifying the impact of “scanxiety.” Lung Cancer. 2016;100:110–113. doi: 10.1016/J.LUNGCAN.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gathinji M, McGirt MJ, Attenello FJ, et al. Association of preoperative depression and survival after resection of malignant brain astrocytoma. Surg Neurol. 2009;71(3):299–303. doi: 10.1016/j.surneu.2008.07.016 [DOI] [PubMed] [Google Scholar]
- 18.Mainio A, Hakko H, Timonen M, Niemelä A, Koivukangas J, Räsänen P. Depression in relation to survival among neurosurgical patients with a primary brain tumor: A 5-year follow-up study. Neurosurgery. 2005;56(6):1234–1241. doi: 10.1227/01.NEU.0000159648.44507.7F [DOI] [PubMed] [Google Scholar]
- 19.Rooney AG, McNamara S, Mackinnon M, et al. Frequency, clinical associations, and longitudinal course of major depressive disorder in adults with cerebral glioma. Journal of Clinical Oncology. 2011;29(32):4307–4312. doi: 10.1200/JCO.2011.34.8466 [DOI] [PubMed] [Google Scholar]
- 20.Litofsky NS, Farace E, Anderson F, et al. Depression in Patients with High-grade Glioma: Results of the Glioma Outcomes Project. Neurosurgery. 2004;54(2):358–367. doi: 10.1227/01.NEU.0000103450.94724.A2 [DOI] [PubMed] [Google Scholar]
- 21.Rooney AG, Brown PD, Reijneveld JC, Grant R. Depression in glioma: A primer for clinicians and researchers. J Neurol Neurosurg Psychiatry. 2014;85(2):230–235. doi: 10.1136/jnnp-2013-306497 [DOI] [PubMed] [Google Scholar]
- 22.Shi C, Lamba N, Zheng LJ, et al. Depression and survival of glioma patients: A systematic review and meta-analysis. Clin Neurol Neurosurg. 2018;172:8–19. doi: 10.1016/j.clineuro.2018.06.016 [DOI] [PubMed] [Google Scholar]
- 23.Keng A, Stewart DE, Sheehan KA. Examining the Neuropsychiatric Sequelae Postsurgical Resection of Adult Brain Tumors Through a Scoping Review. Psychosomatics. 2020;61(3):209–219. doi: 10.1016/j.psym.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 24.Pelletier G, Verhoef MJ, Khatri N, Hagen N. Quality of life in brain tumor patients: The relative contributions of depression, fatigue, emotional distress, and existential issues. J Neurooncol. 2002;57(1):41–49. doi: 10.1023/A:1015728825642 [DOI] [PubMed] [Google Scholar]
- 25.Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: A systematic review of observational studies. J Natl Cancer Inst. 2011;103(1):61–76. doi: 10.1093/jnci/djq458 [DOI] [PubMed] [Google Scholar]
- 26.Rumalla K, Lin M, Orloff E, et al. Effect of Comorbid Depression on Surgical Outcomes After Craniotomy for Malignant Brain Tumors: A Nationwide Readmission Database Analysis. World Neurosurg. 2020;142:e458–e473. doi: 10.1016/j.wneu.2020.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Yang B, Shao D, et al. Longitudinal association of subjective prospective and retrospective memory and depression among patients with glioma. European Journal of Oncology Nursing. 2019;42:1–6. doi: 10.1016/j.ejon.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 28.Mainio A, Hakko H, Niemelä A, Koivukangas J, Räsänen P. Depression and functional outcome in patients with brain tumors: A population-based 1-year follow-up study. J Neurosurg. 2005;103(5):841–847. doi: 10.3171/JNS.2005.103.5.0841 [DOI] [PubMed] [Google Scholar]
- 29.Reinert C, Gerken M, Rathberger K, et al. Single-institution cross-sectional study to evaluate need for information and need for referral to psychooncology care in association with depression in brain tumor patients and their family caregivers. BMC Psychol. 2020;8(1):96. doi: 10.1186/s40359-020-00460-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mainio A, Hakko H, Niemelä A, Tuurinkoski T, Koivukangas J, Räsänen P. The effect of brain tumour laterality on anxiety levels among neurosurgical patients. J Neurol Neurosurg Psychiatry. 2003;74(9):1278–1282. doi: 10.1136/jnnp.74.9.1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wellisch DK, Kaleita TA, Freeman D, Cloughesy T, Goldman J. Predicting major depression in brain tumor patients. Psychooncology. 2002;11(3):230–238. doi: 10.1002/pon.562 [DOI] [PubMed] [Google Scholar]
- 32.Chang SM, Parney IF, McDermott M, et al. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg. 2003;98(6):1175–1181. doi: 10.3171/jns.2003.98.6.1175 [DOI] [PubMed] [Google Scholar]
- 33.Pidani AS, Siddiqui AR, Azam I, Shamim MS, Jabbar AA, Khan S. Depression among adult patients with primary brain tumour: a cross-sectional study of risk factors in a low-middle-income country. BMJ Open. 2020;10(9):e032748. doi: 10.1136/bmjopen-2019-032748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Løppenthin K, Johansen C, Larsen MB, et al. Depressive Symptoms in Danish Patients With Glioma and a Cancer-Free Comparison Group. J Natl Compr Canc Netw. 2020;18(9):1222–1229. doi: 10.6004/jnccn.2020.7570 [DOI] [PubMed] [Google Scholar]
- 35.Park DY, Tom MC, Wei W, et al. Quality of life following concurrent temozolomide-based chemoradiation therapy or observation in low-grade glioma. J Neurooncol. 2022;156(3):499–507. doi: 10.1007/s11060-021-03920-6 [DOI] [PubMed] [Google Scholar]
- 36.Wang Q, Qi F, Song X, et al. A prospective longitudinal evaluation of cognition and depression in postoperative patients with high-grade glioma following radiotherapy and chemotherapy. J Cancer Res Ther. 2018;14(Supplement):S1048–S1051. doi: 10.4103/0973-1482.199431 [DOI] [PubMed] [Google Scholar]
- 37.Acquaye AA, Lin L, Vera-Bolanos E, Gilbert MR, Armstrong TS. Hope and mood changes throughout the primary brain tumor illness trajectory. Neuro Oncol. 2016;18(1):119–125. doi: 10.1093/neuonc/nov101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang SM, Parney IF, Huang W, et al. Patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293(5):557–564. doi: 10.1001/jama.293.5.557 [DOI] [PubMed] [Google Scholar]
- 39.Goebel S, Stark AM, Kaup L, von Harscher M, Mehdorn HM. Distress in patients with newly diagnosed brain tumours. Psychooncology. 2011;20(6):623–630. doi: 10.1002/pon.1958 [DOI] [PubMed] [Google Scholar]
- 40.Bayoumi AB, Efe IE, Ozturk OC, et al. Antidepressant Prescriptions in Neurosurgical Practice: A Survey of Current Trends. Turk Neurosurg. 2019;29(2):289–296. [DOI] [PubMed] [Google Scholar]
- 41.Weiss Lucas C, Renovanz M, Jost J, Sabel M, Wiewrodt D, Rapp M. Assessment Practice of Patient-Centered Outcomes in Surgical Neuro-Oncology: Survey-Based Recommendations for Clinical Routine. Front Oncol. 2021;11:702017. doi: 10.3389/fonc.2021.702017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halkett GKB, Berg MN, Daudu D, et al. Supportive care of patients diagnosed with high grade glioma and their carers in Australia. J Neurooncol. 2022;157(3):475–485. doi: 10.1007/s11060-022-03991-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rydén I, Thurin E, Carstam L, et al. Psychotropic and anti-epileptic drug use, before and after surgery, among patients with low-grade glioma: a nationwide matched cohort study. BMC Cancer. 2021;21(1):248. doi: 10.1186/s12885-021-07939-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keir ST, Farland MM, Lipp ES, Friedman HS. Distress persists in long-term brain tumor survivors with glioblastoma multiforme. Journal of Cancer Survivorship. 2008;2(4):269–274. doi: 10.1007/S11764-008-0069-7 [DOI] [PubMed] [Google Scholar]
- 45.Randazzo DM, McSherry F, Herndon JE, et al. Psychosocial distress and its effects on the health-related quality of life of primary brain tumor patients. Journal of Clinical Oncology. 2015;33(15_suppl):9553–9553. doi: 10.1200/jco.2015.33.15_suppl.9553 [DOI] [Google Scholar]
- 46.Singer S, Roick J, Danker H, et al. Psychiatric co-morbidity, distress, and use of psycho-social services in adult glioma patients-a prospective study. Acta Neurochir. 2018;160(6):1187–1194. doi: 10.1007/s00701-018-3527-7 [DOI] [PubMed] [Google Scholar]
- 47.Randazzo DM, McSherry F, Herndon JE, et al. A cross sectional analysis from a single institution’s experience of psychosocial distress and health-related quality of life in the primary brain tumor population. J Neurooncol. 2017;134(2):363–369. doi: 10.1007/s11060-017-2535-4 [DOI] [PubMed] [Google Scholar]
- 48.Kvale EA, Murthy R, Taylor R, Lee JY, Nabors LB. Distress and quality of life in primary high-grade brain tumor patients. Supportive Care in Cancer. 2009;17(7):793–799. doi: 10.1007/s00520-008-0551-9 [DOI] [PubMed] [Google Scholar]
- 49.Keir ST, Guill AB, Carter KE, Friedman HS. Stress and intervention preferences of patients with brain tumors. Supportive Care in Cancer. 2006;14(12):1213–1219. doi: 10.1007/s00520-006-0087-9 [DOI] [PubMed] [Google Scholar]
- 50.Keir ST, Swartz JJ, Friedman HS. Stress and long-term survivors of brain cancer. Supportive Care in Cancer. 2007;15(12):1423–1428. doi: 10.1007/s00520-007-0292-1 [DOI] [PubMed] [Google Scholar]
- 51.Palese A, Cecconi M, Moreale R, Skrap M. Pre-operative stress, anxiety, depression and coping strategies adopted by patients experiencing their first or recurrent brain neoplasm: An explorative study. Stress and Health. 2012;28(5):416–425. doi: 10.1002/smi.2472 [DOI] [PubMed] [Google Scholar]
- 52.Jiang C, Wang J. Post-traumatic stress disorders in patients with low-grade glioma and its association with survival. J Neurooncol. 2019;142(2):385–392. doi: 10.1007/S11060-019-03112-3 [DOI] [PubMed] [Google Scholar]
- 53.Gibson AW, Graber JJ. Distinguishing and treating depression, anxiety, adjustment, and post-traumatic stress disorders in brain tumor patients. Ann Palliat Med. 2021;10(1):875–892. doi: 10.21037/apm-20-509 [DOI] [PubMed] [Google Scholar]
- 54.Fehrenbach MK, Brock H, Mehnert-Theuerkauf A, Meixensberger J. Psychological Distress in Intracranial Neoplasia: A Comparison of Patients With Benign and Malignant Brain Tumours. Front Psychol. 2021;12. doi: 10.3389/fpsyg.2021.664235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruce M, Gumley D, Isham L, Fearon P, Phipps K. Post-traumatic stress symptoms in childhood brain tumour survivors and their parents. Child Care Health Dev. 2011;37(2):244–251. doi: 10.1111/j.1365-2214.2010.01164.x [DOI] [PubMed] [Google Scholar]
- 56.Szpringer M, Oledzka M, Amann BL. A Non-randomized Controlled Trial of EMDR on Affective Symptoms in Patients With Glioblastoma Multiforme. Front Psychol. 2018;9:785. doi: 10.3389/fpsyg.2018.00785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao X Reminiscence therapy-based care program for reducing anxiety and depression in glioma survivors: A randomized controlled trial. Medicine. 2021;100(5):e23056. doi: 10.1097/MD.0000000000023056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boele FW, Rooney AG, Grant R, Klein M. Psychiatric symptoms in glioma patients: From diagnosis to management. Neuropsychiatr Dis Treat. 2015;11:1413–1420. doi: 10.2147/NDT.S65874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robertson ME, McSherry F, Herndon JE, Peters KB. Insomnia and its associations in patients with recurrent glial neoplasms. Springerplus. 2016;5(1). doi: 10.1186/S40064-016-2578-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lynam LM, Lyons MK, Drazkowski JF, et al. Frequency of seizures in patients with newly diagnosed brain tumors: A retrospective review. Clin Neurol Neurosurg. 2007;109(7):634–638. doi: 10.1016/j.clineuro.2007.05.017 [DOI] [PubMed] [Google Scholar]
- 61.Belcastro V, Pisani LR, Bellocchi S, et al. Brain tumor location influences the onset of acute psychiatric adverse events of levetiracetam therapy: an observational study. J Neurol. 2017;264(5):921–927. doi: 10.1007/s00415-017-8463-6 [DOI] [PubMed] [Google Scholar]
- 62.Bernett A, Phenis R, Fonkem E, Aceves J, Kirmani B, Cruz-Laureano D. Neurobehavioral effects of levetiracetam in brain tumor related epilepsy. Front Neurol. 2013;4 JUL. doi: 10.3389/fneur.2013.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Groot M, Aronica E, Heimans JJ, Reijneveld JC. Synaptic vesicle protein 2A predicts response to levetiracetam in patients with glioma. Neurology. 2011;77(6):532–539. doi: 10.1212/WNL.0b013e318228c110 [DOI] [PubMed] [Google Scholar]
- 64.van der Meer PB, Koekkoek JAF, van den Bent MJ, Dirven L, Taphoorn MJB. Effect of antiepileptic drugs in glioma patients on self-reported depression, anxiety, and cognitive complaints. J Neurooncol. 2021;153(1):89–98. doi: 10.1007/s11060-021-03747-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pan-Weisz TM, Kryza-Lacombe M, Burkeen J, Hattangadi-Gluth J, Malcarne VL, McDonald CR. Patient-reported health-related quality of life outcomes in supportive-care interventions for adults with brain tumors: A systematic review. Psychooncology. 2019;28(1):11–21. doi: 10.1002/pon.4906 [DOI] [PubMed] [Google Scholar]
- 66.Ownsworth T, Chambers S, Damborg E, Casey L, Walker DG, Shum DHK. Evaluation of the making sense of brain tumor program: A randomized controlled trial of a home-based psychosocial intervention. Psychooncology. 2015;24(5):540–547. doi: 10.1002/pon.3687 [DOI] [PubMed] [Google Scholar]
- 67.Dinapoli L, Chiesa S, Dinapoli N, et al. Personalised support of brain tumour patients during radiotherapy based on psychological profile and quality of life. Supportive Care in Cancer. 2021;29(8):4555–4563. doi: 10.1007/s00520-021-06000-7 [DOI] [PubMed] [Google Scholar]
- 68.Eisenhut L, Sadeghi-Bahmani D, Gerber M, et al. Effects of two types of exercise training on psychological well-being, sleep and physical fitness in patients with high-grade glioma (WHO III and IV). J Psychiatr Res. 2022;151:354–364. doi: 10.1016/j.jpsychires.2022.03.058 [DOI] [PubMed] [Google Scholar]
- 69.Mohr DC, Vella L, Hart S, Heckman T, Simon G. The effect of telephone-administered psychotherapy on symptoms of depression and attrition: A meta-analysis. Clinical Psychology: Science and Practice. 2008;15(3):243–253. doi: 10.1111/j.1468-2850.2008.00134.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones S, Ownsworth T, Shum DHK. Feasibility and utility of telephone-based psychological support for people with brain tumor: A single-case experimental study. Front Oncol. 2015;5. doi: 10.3389/fonc.2015.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beevers Z, Hussain S, Boele… FW. Pharmacological treatment of depression in people with a primary brain tumour. The Cochrane Database …. Published online 2020. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7388852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caudill JS, Brown PD, Cerhan JH, Rummans TA. Selective serotonin reuptake inhibitors, glioblastoma multiforme, and impact on toxicities and overall survival: The mayo clinic experience. American Journal of Clinical Oncology: Cancer Clinical Trials. 2011;34(4):385–387. doi: 10.1097/COC.0b013e3181e8461a [DOI] [PubMed] [Google Scholar]
- 73.Otto-Meyer S, DeFaccio R, Dussold C, et al. A retrospective survival analysis of Glioblastoma patients treated with selective serotonin reuptake inhibitors. Brain Behav Immun Health. 2020;2:100025. doi: 10.1016/j.bbih.2019.100025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rabin EE, Kim M, Mozny A, et al. A systematic review of pharmacologic treatment efficacy for depression in older patients with cancer. Brain Behav Immun Health. 2022;21:100449. doi: 10.1016/j.bbih.2022.100449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pu B, Wang N, Wang C, Sun B. Clinical observation on the benefits of antidepressant intervention in advanced cancer patients. Medicine. 2022;101(26). doi: 10.1097/MD.0000000000029771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Villa GR, Hulce JJ, Zanca C, et al. An LXR-Cholesterol Axis Creates a Metabolic Co-Dependency for Brain Cancers. Cancer Cell. 2016;30(5):683–693. doi: 10.1016/j.ccell.2016.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doherty GJ, de Paula BHR. Cannabinoids in glioblastoma multiforme—hype or hope? Br J Cancer. 2021;124(8):1341–1343. doi: 10.1038/s41416-021-01265-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Twelves C, Sabel M, Checketts D, et al. A phase 1b randomised, placebo-controlled trial of nabiximols cannabinoid oromucosal spray with temozolomide in patients with recurrent glioblastoma. Br J Cancer. 2021;124(8):1379–1387. doi: 10.1038/s41416-021-01259-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kleckner AS, Kleckner IR, Kamen CS, et al. Opportunities for cannabis in supportive care in cancer. Ther Adv Med Oncol. 2019;11. doi: 10.1177/1758835919866362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Finocchiaro CY, Petruzzi A, Lamperti E, et al. The burden of brain tumor: A single-institution study on psychological patterns in caregivers. J Neurooncol. 2012;107(1):175–181. doi: 10.1007/s11060-011-0726-y [DOI] [PubMed] [Google Scholar]
- 81.Sterckx W, Coolbrandt A, Dierckx de Casterlé B, et al. The impact of a high-grade glioma on everyday life: A systematic review from the patient’s and caregiver’s perspective. European Journal of Oncology Nursing. 2013;17(1):107–117. doi: 10.1016/j.ejon.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 82.Sherwood PR, Given BA, Given CW, et al. Predictors of distress in caregivers of persons with a primary malignant brain tumor. Res Nurs Health. 2006;29(2):105–120. doi: 10.1002/nur.20116 [DOI] [PubMed] [Google Scholar]
- 83.Chen C, Wang H, Zhang L, et al. Clinical study of preoperative psychological distress and its related factors in the primary caregivers of patients with glioma. Clin Neurol Neurosurg. 2021;200:106364. doi: 10.1016/j.clineuro.2020.106364 [DOI] [PubMed] [Google Scholar]
- 84.Forst DA, Podgurski AF, Quain KM, et al. Factors associated with psychological distress in caregivers of patients with malignant gliomas. Support Care Cancer. 2022;30(7):5811–5820. doi: 10.1007/s00520-022-06989-5 [DOI] [PubMed] [Google Scholar]
- 85.Jansson MRN, von Heymann-Horan A, Rasmussen BK, et al. Risk for use of antidepressants, anxiolytics, and hypnotics in partners of glioma patients-A nationwide study covering 19 years of prescriptions. Psychooncology. 2018;27(8):1930–1936. doi: 10.1002/pon.4744 [DOI] [PubMed] [Google Scholar]
- 86.Janda M, Steginga S, Dunn J, Langbecker D, Walker D, Eakin E. Unmet supportive care needs and interest in services among patients with a brain tumour and their carers. Patient Educ Couns. 2008;71(2):251–258. doi: 10.1016/j.pec.2008.01.020 [DOI] [PubMed] [Google Scholar]
- 87.Milbury K, Li J, Weathers SP, et al. Pilot randomized, controlled trial of a dyadic yoga program for glioma patients undergoing radiotherapy and their family caregivers. Neurooncol Pract. 2019;6(4):311–320. doi: 10.1093/nop/npy052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hao A, Huang J, Xu X. Anxiety and depression in glioma patients: prevalence, risk factors, and their correlation with survival. Ir J Med Sci. 2021;190(3):1155–1164. doi: 10.1007/s11845-020-02374-5 [DOI] [PubMed] [Google Scholar]
- 89.Mukand JA, Blackinton DD, Crincoli MG, Lee JJ, Santos BB. Incidence of neurologic deficits and rehabilitation of patients with brain tumors. Am J Phys Med Rehabil. 2001;80(5):346–350. doi: 10.1097/00002060-200105000-00005 [DOI] [PubMed] [Google Scholar]
- 90.Mayer S, Fuchs S, Fink M, et al. Hope and Distress Are Not Associated With the Brain Tumor Stage. Front Psychol. 2021;12. doi: 10.3389/FPSYG.2021.642345 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.