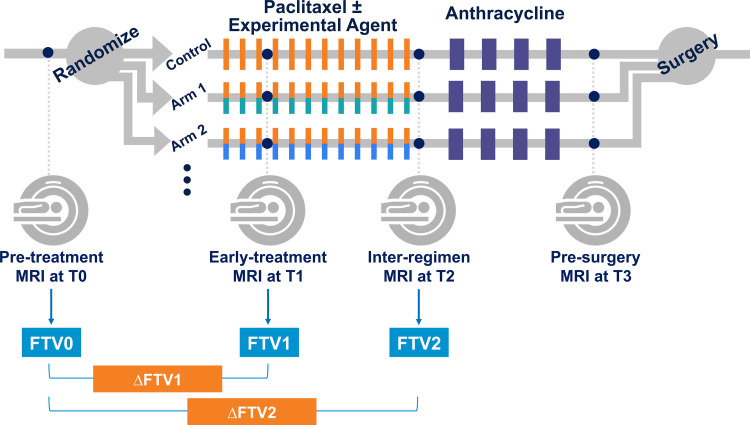
Figure 1:
Schematic shows study protocol. Participants were randomly assigned to one of 10 neoadjuvant drug arms (nine experimental drug arms and one standard-of-care control arm). Each participant underwent MRI examination at four treatment time points (T0, T1, T2, T3) during neoadjuvant chemotherapy. FTV0, FTV1, FTV2 = functional tumor volume at T0, T1, and T2, respectively; ΔFTV1, ΔFTV2 = percentage change of functional tumor volume relative to T0 at T1 and T2, respectively.