Abstract
Fetal alcohol spectrum disorder (FASD) has been linked to numerous poor neurological outcomes as well as impairments in respiratory neural control. Females are known to metabolize ethanol (EtOH) differently than males suggesting a sexual dimorphic sensitivity to EtOH exposure. We used a rodent model of FASD to investigate whether EtOH disrupts respiratory neural control. Rat pups received a single intraperitoneal injection of 2 different doses (0.8 mg/g or 4.4 mg/g) of EtOH. Whole-body plethysmography was used ~24 h later to assess ventilatory responses to acute hypoxia (HVR) and hypercapnia (HCVR). Females treated with 4.4 mg/g of EtOH exhibited an attenuated HVR and HCVR, but there was no effect on males, and no effect of 0.8mg/g on either sex. There was unexpected mortality of unknown causes, especially in females, that occurred 2–3 days after EtOH administration. These data suggest that important ventilatory defense responses in females are impaired following developmental EtOH exposure, and this may be associated with increased risk of later death.
Keywords: Fetal Alcohol Spectrum Disorder (FASD), hypoxia, hypercapnia, respiratory control, plethysmography, Sudden Unexpected Infant Death (SUID)
1.0. Introduction:
Exposure to ethanol (EtOH, the alcohol in alcoholic beverages) is known to have a wide range of serious detrimental effects on fetal and postnatal development, resulting in intrauterine growth retardation, congenital malformations and long-term cognitive impairment. Despite the known dangers of EtOH consumption in pregnancy, fetuses are still put at risk of exposure to EtOH. Fetal exposure can lead to premature birth, low birthweight and, in severe cases, admission to the Neonatal Intensive Care Unit (NICU). Preterm or term neonates who spend time in the NICU can also be exposed to EtOH via hand sanitizer and/or excipient administration in the NICU (Stefanak et al., 2020). Animal models of Fetal Alcohol Spectrum Disorder (FASD) have shown in utero EtOH exposure decreases growth of the fetus and their lungs are smaller and have less DNA (Inselman et al., 1985). Fetal EtOH exposure also reduces mitochondrial activity and proliferation, with excessive apoptosis in the cerebra and the cerebellum (Xu et al., 2005), which would be consistent with neurodevelopmental impairments.
Beyond the fetal period, EtOH exposure can also increase the risk of life threatening events, including Sudden Unexpected Infant Death (SUID) (Myers et al., 2017), which could suggest adverse effects on respiratory and autonomic function. In utero EtOH exposure decreased fetal breathing movements (Lewis and Boylan et al., 1979) and longer-term effects included respiratory depression (Dubois et al., 2013). In a rat model of Neonatal Opioid Withdrawal Syndrome, neonates expressed tachypnea associated with spontaneous withdrawal and impaired ventilatory defense responses to acute hypoxia (HVR) and hypercapnia (HCVR) (Osborne et al., 2022). Infants born to known substance-abusing mothers were more prone to periodic breathing with exposure to hypoxia, were less likely to arouse from hypercapnia, had more apnea and abnormal sleeping patterns, and were more prone to SUID compared to control infants (Ward et al., 1992). Impairments in respiratory neural control following developmental exposure to substances of abuse could be an underlying feature of arousal deficits in SUID cases. Finally, there is evidence that EtOH and other misused substances have varied effects on the respective sexes, such as how males metabolize EtOH differently than females (Uban et al., 2017). The consequences of EtOH exposure on respiratory neural control and whether there are sex-specific effects have not yet been thoroughly studied. In the present study, therefore, we used a rodent model of preterm EtOH exposure to investigate sex-specific effects on respiratory control.
2.0. Methods
2.1. Ethical Approval:
Experiments were performed on neonatal Sprague-Dawley rats (Envigo) maintained under standard housing conditions on a 12:12 h light:dark cycle. All procedures were carried out in accordance with the National Institute of Health guidelines for care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee at Case Western Reserve University.
2.2. Ethanol exposure:
Postnatal-day (P) 5 female and male Sprague-Dawley rat pups were removed from the dam and injected intraperitoneally with either 0.8 or 4.4 mg/g of EtOH in a solution of 10 μl/g of body weight (pH 7.6 & 8.1, respectively), or an equal volume of phosphate buffered saline (PBS, pH 7.4). Pups were randomized to treatment groups by an independent investigator, and measurements of whole-body plethysmography were performed by a different blinded investigator. The ventilatory responses to acute hypoxia (HVR; 10% O2, 5 min) and hypercapnia (HCVR; 5% CO2, 5 min) were measured using wholebody plethysmography 24 h later on P6. Rats were returned to the dam for normal rearing until weaning (~3 weeks of age). Minute-by-minute changes in ventilation during hypoxic and hypercapnic challenge (expressed as a delta change from baseline normoxic ventilation) were used as indices of respiratory control. Comparisons were made between PBS and EtOH injected rats.
2.3. Whole-Body Plethysmography:
Rats were removed from the litter on P6, between 22 and 28 h after treatment on P5, and placed inside a custom-made plethysmograph chamber. Airflow through the chamber was held constant using a mass flow controller (Aalborg, NY, USA). Temperature inside the chamber was maintained (~28–30° C) by adjusting a water bath (Isotemp 3013S, Fisher Scientific; PA, USA) that circulated water to a heat pad positioned underneath the plethysmograph. The chamber was assessed for adequate seal by observing the stability of the square pressure change following injection of a calibration volume (50 μl) using a glass micro-syringe (Hamilton, Harvard Apparatus; MA, USA). The same injection volume was used later for calibration of tidal volume changes associated with breathing. Rectal temperature was measured at the end of the experiment with a fine-temperature thermocouple (Physitemp; NJ, USA). Rats were allowed approximately 60 minutes to acclimate to the plethysmograph before being exposed to acute hypoxia for the HVR. Rats were then allowed a five-minute recovery period of normoxia before receiving five minutes of hypercapnia for measurement of the HCVR. Measurements of minute ventilation (VE), frequency (fR) and tidal volume (VT) were made during baseline, and during each minute of hypoxia and hypercapnia. Ventilation was measured when the plethysmograph was sealed for ~20 seconds at each minute of exposure to the test gas. Chambers were sealed by turning stopcocks upstream and downstream of the plethysmograph. The corresponding pressure signal was associated with breathing during the time the chamber was sealed and was calibrated to calculate tidal volume. The HVR was further delineated into the early (1st−2nd minute) and late (4th −5th minute) phase to characterize a biphasic HVR.
2.4. Statistical analysis:
Statistical comparisons of plethysmography measurements were made between treatment groups using Two-Way repeated measures ANOVA and a Student Newman-Keuls post-hoc analysis. Differences were considered significant at P< 0.05. All values are expressed as mean ± 1 SEM. Kaplan-Meier curves were plotted to assess survival.
3.0. Results
3.1. Hypoxic and Hypercapnic ventilatory responses:
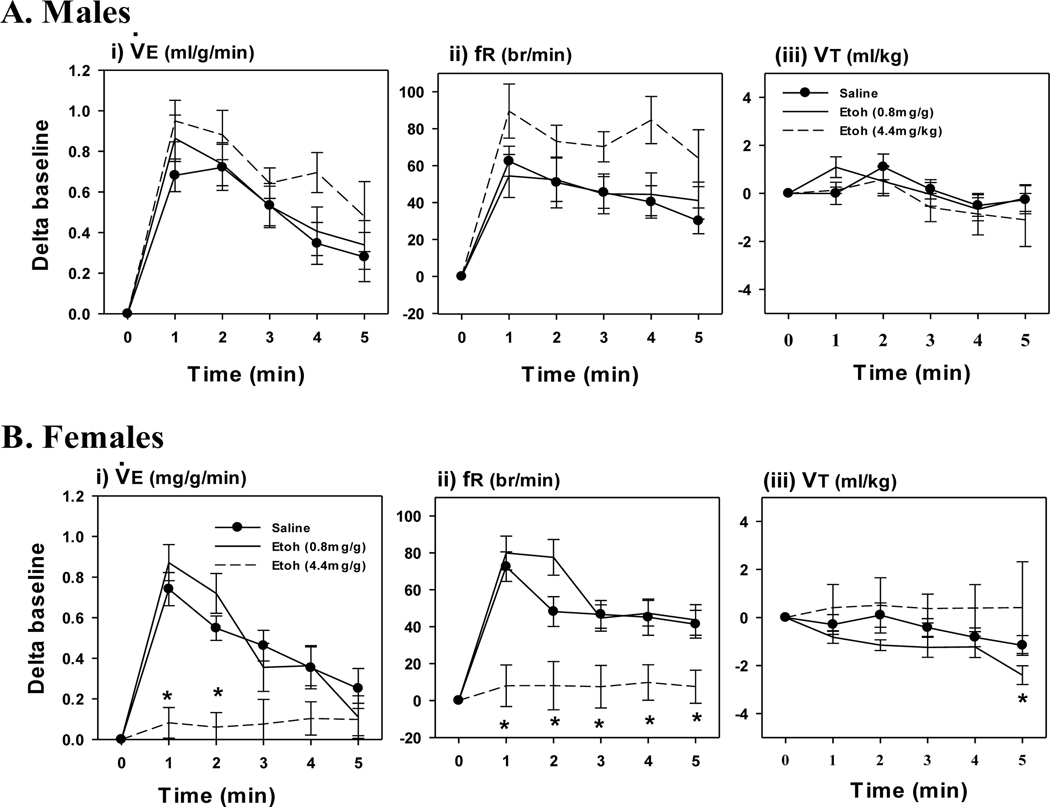
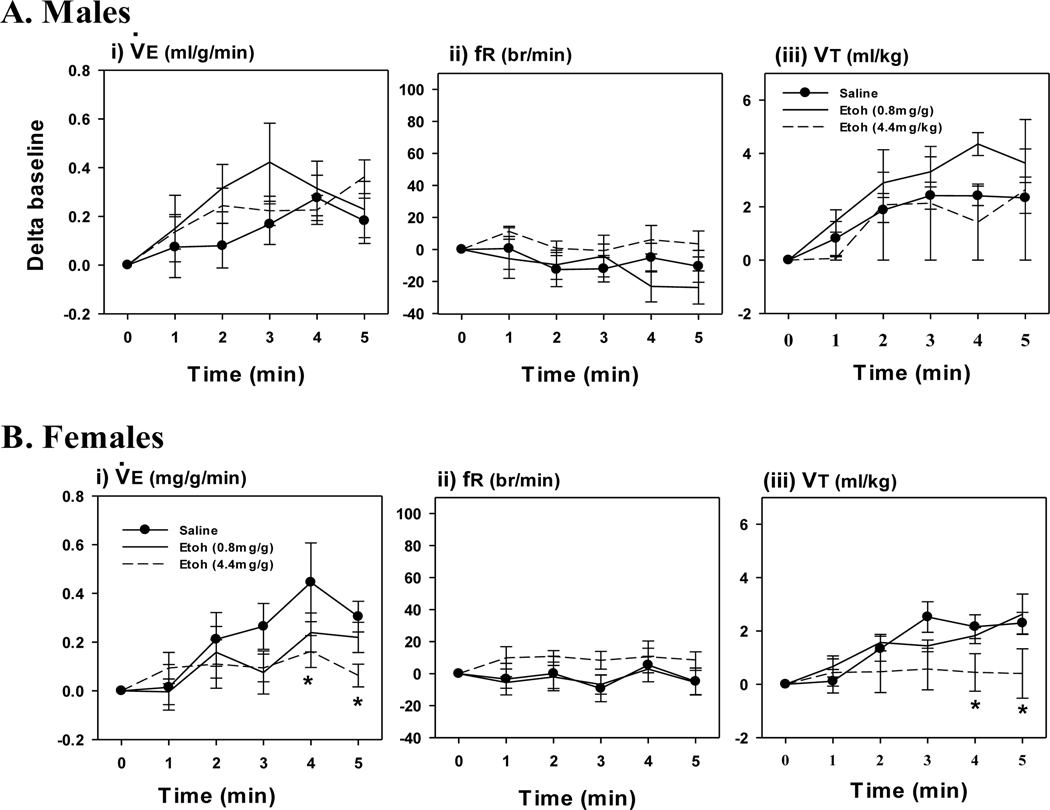
EtOH exposure did not affect the acute HVR in EtOH treated males compared to PBS, although there was a tendency for an increased fR response at the highest dose (4.4 mg/g; Fig. 1A). In contrast, the highest (but not lowest, 0.8 mg/g) dose of EtOH significantly attenuated the HVR in females (Fig. 1B). The attenuated HVR in the females resulted from a decreased fR, whereas VT was not affected. EtOH had a similar sex-specific effect on the HCVR. EtOH did not affect the HCVR at either dose in males (Fig. 2A), whereas the highest dose significantly attenuated the HCVR in females (Fig. 2B). The attenuated HCVR in females was mostly through a reduction in the VT response whereas fR was not affected compared to PBS.
Fig. 1. Response to Acute Hypoxia.
Minute Ventilation (I, ), frequency (ii, fR) and tidal volume (iii, VT) responses to acute hypoxia (i-iii) in P6 day old male (A) and female (B) rats, 24 hours after an intraperitoneal injection of EtOH (0.8 mg/g or 4.4 mg/g). Control rats were injected with PBS. Note, the highest dose of EtOH (open circles, dashed line) attenuated the acute HVR in females but not males. Values are expressed as means ± 1 S.E.M. *indicates significant difference PBS treated rats (<0.05).
Fig. 2. Response to Acute Hypercapnia.
Minute Ventilation (I, ), frequency (ii, fR) and tidal volume (iii, VT) responses to acute hypercapnia (i-iii) in P6 day old male (A) and female (B) rats, 24 hours after an intraperitoneal injection of EtOH (0.8 mg/g or 4.4 mg/g). Control rats were injected with PBS. Note, the highest dose of EtOH (open circles, dashed line) attenuated the HCVR in females but not males. Values are expressed as means ± 1 S.E.M. *indicates significant difference PBS treated rats (<0.05).
3.2. Kaplan Meier Curves:
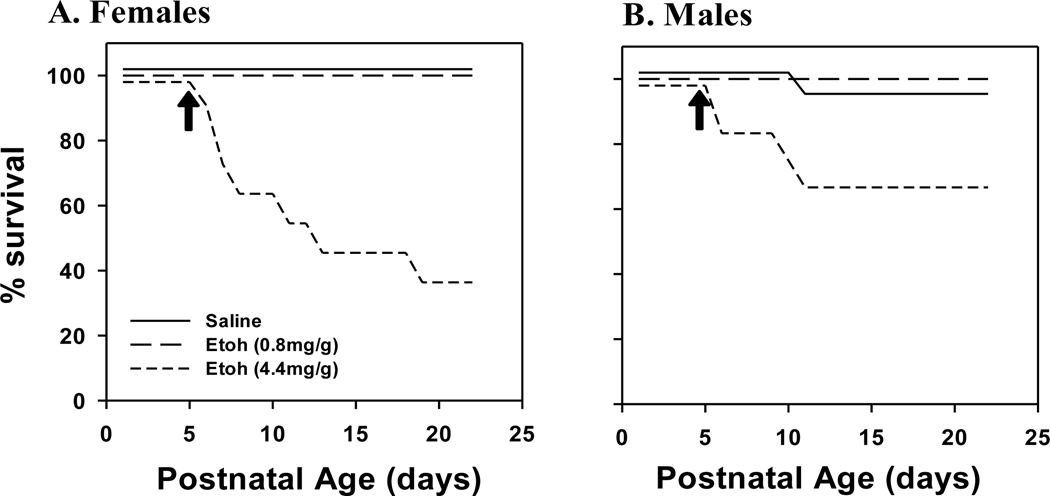
Following plethysmography, pups were returned to the nursing dam until weaning. However, we noted a significant rate of mortality in pups that received the higher dose of EtOH. Kaplan Meier Curves were constructed to document the timeline of mortality in both male and female rats (Fig. 3). Mortality occurred only at the highest dose of EtOH (4.4 mg/g) in both sexes. Most of the animals who died did so within 5 days of the EtOH injection. Mortality was highest in females with 64% of pups (7 deaths out of 11 injected pups) having expired by 23 days of age (18 days post-injection; Fig. 3A). Mortality was lower in males with 33% of pups (4 deaths out of 12 injected pups) having expired by 23 days of postnatal age (Fig. 3B).
Fig. 3. Kaplan-Meier Curves.
Kaplan-Meier curves in female (A) and male (B) rats following intraperitoneal injection of EtOH (0.8 mg/g or 4.4 mg/g). Note the precipitous rate of mortality 2–5 days after birth, especially in females (A). Rats were monitored for mortality until day of life 23. *indicates significant difference PBS treated rats (<0.05). N-number: Females, Saline = 23, 0.8 mg/g EtOH = 10, 4.4 mg/g EtOH = 11; Males, Saline = 22, 0.8 mg/g EtOH = 13, 4.4 mg/g EtOH = 12.
Discussion:
FASD has a known array of serious detriments, including physical defects of the face, eyes and ears, accompanied by impairments in vision and hearing, learning disorders, communication and social difficulties, and impairments to decision-making and problem-solving. There is some evidence that FASD also affects the respiratory system. Studies have shown that prenatal EtOH exposure can lead to lung growth restriction (Fatayerji et al., 1996; Inselman et al., 1985). In addition to the anatomical effects on the lung, EtOH exposure in utero in animals has been shown to impair the action of insulin-like growth factors (IGFs), as well as the production of surfactant (Fatayerji et al., 1996). However, not a lot is known about the effects of EtOH on the development of respiratory neural control. A prior study demonstrated chronic postnatal EtOH exposure augmented the HVR in 9 day old rats, which was suggestive of sensitization and neuroadaptive changes in central respiratory networks controlling breathing (Macchione et al., 2021). In the present study, we showed a single (acute) bolus injection of EtOH in P5 day old rats abolished the acute HVR 24 h post-treatment in females only. The discrepancies between these findings could be related to the degree (acute v chronic) and timing of EtOH treatment; sex is likely also a consideration since the study by Macchione did not compare males with females.
We also showed that females were more vulnerable to EtOH exposure than males, as indicated by impairments in the acute HVR and HCVR, and higher mortality. Early postnatal development of respiratory neural control is well understood in rats, and the specific effects on the early phase of the HVR (1st−2nd minute) in the females could reflect a peripheral effect on respiratory control mechanisms. However, a central effect of EtOH should not be ruled out, especially since the HCVR was also attenuated (in females). These data are consistent with impaired ventilatory responses seen in infants born from mothers of substance use/misuse (Ward et al., 1992), although that study was not powered to assess a sex-specific comparison and the specific substance of use/misuse causing the respiratory impairments was not known.
There are also sex-specific effects of EtOH exposure, which are likely due to sex hormones and differences in abilities to metabolize EtOH between males and females (Uban et al., 2017). Studies have shown differences in white matter microstructure between females and males exposed prenatally to EtOH (Uban et al., 2017). Males have lower viability and increased mortality by first grade, while females show more dysmorphology and neurocognitive impairment, which results in a likelier diagnosis of FASD for females (May et al., 2017). The higher mortality rate (compared to males) seen in the females of the present study is also of significant concern. It is generally accepted that males are more vulnerable to SUID, although the reasons why are poorly understood. Males account for about 60% of SUID cases (Mage et al., 2006) and it has been proposed that the 1/3 lower mortality for females is due to an X-linked allele occurring at a frequency of 1/3. The allele has been proposed to offer females a slightly higher resistance to hypoxia compared to males, which could be life-saving in some cases. This might also explain why females are less susceptible to Infant Respiratory Distress Syndrome and other forms of suffocation (Mage et al., 2006). In this study we have shown the opposite effect, whereby females had a significantly increased likelihood of mortality associated with impaired HVR and HCVR compared to males. This could indicate that prenatal alcohol consumption could be a greater risk factor for females than males in SUID. Future studies are needed to understand the breadth and severity of the effects of EtOH consumption during pregnancy, the mechanisms underlying consequential respiratory dysfunction, the different vulnerabilities between males and females, and how these may be linked to SUID.
Highlights:
This study uses a rat model of Fetal Alcohol Spectrum Disorder (FASD) to investigate the effects of neonatal ethanol exposure on respiratory neural control.
A single intraperitoneal injection of ethanol dose-dependently attenuated the acute hypoxic and hypercapnic ventilatory response in female but not male rats.
Ethanol also caused an unexpected precipitous rate of mortality among female pups starting a few days after injection.
These data highlight the vulnerability of important ventilatory defense mechanisms, and reveal sex disparities in the vulnerability of these mechanisms following developmental ethanol exposure.
Acknowledgments
Statement of financial support: This study was funded by the NIH/NICHD R21HD085061.
Footnotes
Category of Study: Basic Science
Author Statement
Nicholas Rickman: Methodology, Investigation, Software, Resources, Project Administration, Formal Analysis, Data Curation, Visualization, Writing - Original Draft
Catherine Mayer: Methodology, Validation, Investigation, Software, Resources, Supervision, Project Administration, Writing - Review & Editing
Sergei Vatolin: Methodology, Validation, Resources, Supervision, Project Administration, Writing - Review & Editing
Cansu Tokat: Validation, Investigation, Resources, Writing - Review & Editing
Mrinaj Janampalli: Validation, Investigation, Resources, Writing - Review & Editing
Cynthia Bearer: Conceptualization, Methodology, Validation, Resources, Supervision, Project Administration, Formal Analysis, Visualization, Funding Acquisition, Writing - Review & Editing
Peter MacFarlane: Conceptualization, Methodology, Validation, Investigation, Software, Resources, Supervision, Project Administration, Formal Analysis, Data Curation, Visualization, Funding Acquisition, Writing - Review & Editing
Disclosure: The authors declare no conflict of interest in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Stefanak MP, Al-Mudares F, El-Metwally D, Jones JW, Kane MA, Bearer CF. High concentrations of urinary ethanol metabolites in neonatal intensive care unit infants. Pediatr Res. 2020. Dec;88(6):865–870. doi: 10.1038/s41390-020-1020-5. Epub 2020 Jun 20. PMID: 32563185. [DOI] [PubMed] [Google Scholar]
- 2.Inselman LS, Fisher SE, Spencer H, Atkinson M. Effect of intrauterine ethanol exposure on fetal lung growth. Pediatr Res. 1985. Jan;19(1):12–4. doi: 10.1203/00006450-198501000-00004. PMID: 2578634. [DOI] [PubMed] [Google Scholar]
- 3.Myers MM, Elliott AJ, Odendaal HJ, Burd L, Angal J, Groenewald C, Nugent JD, Yang JS, Isler JR, Dukes KA, Robinson F, Fifer WP; PASS Network. Cardiorespiratory physiology in the safe passage study: protocol, methods and normative values in unexposed infants. Acta Paediatr. 2017. Aug;106(8):1260–1272. doi: 10.1111/apa.13873. Epub 2017 May 15. PMID: 28419567; PMCID: PMC5530586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Y, Liu P, Li Y. Impaired development of mitochondria plays a role in the central nervous system defects of fetal alcohol syndrome. Birth Defects Res A Clin Mol Teratol. 2005. Feb;73(2):83–91. doi: 10.1002/bdra.20110. PMID: 15690350. [DOI] [PubMed] [Google Scholar]
- 5.Uban KA, Herting MM, Wozniak JR, Sowell ER; CIFASD. Sex differences in associations between white matter microstructure and gonadal hormones in children and adolescents with prenatal alcohol exposure. Psychoneuroendocrinology. 2017. Sep;83:111–121. doi: 10.1016/j.psyneuen.2017.05.019. Epub 2017 May 26. PMID: 28609669; PMCID: PMC5877456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osborne A, Mayer CA, Hoffman A, Cali V, Hyzny R, Lewis SJ, MacFarlane PM. Cardiorespiratory anomalies and increased brainstem microglia in a rat model of neonatal opioid withdrawal syndrome.Respir. Physiol. Neurobiol 2022. 296: 103800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward SL, Bautista DB, Woo MS, Chang M, Schuetz S, Wachsman L, Sehgal S, Bean X. Responses to hypoxia and hypercapnia in infants of substance-abusing mothers. J Pediatr. 1992. Nov;121(5 Pt 1):704–9. doi: 10.1016/s0022-3476(05)81896-7. PMID: 1432417. [DOI] [PubMed] [Google Scholar]
- 8.Fatayerji N, Engelmann GL, Myers T, Handa RJ. In utero exposure to ethanol alters mRNA for insulin-like growth factors and insulin-like growth factor-binding proteins in placenta and lung of fetal rats. Alcohol Clin Exp Res. 1996. Feb;20(1):94–100. doi: 10.1111/j.1530-0277.1996.tb01051.x. PMID: 8651471. [DOI] [PubMed] [Google Scholar]
- 9.May PA, Tabachnick B, Hasken JM, Marais AS, de Vries MM, Barnard R, Joubert B, Cloete M, Botha I, Kalberg WO, Buckley D, Burroughs ZR, Bezuidenhout H, Robinson LK, Manning MA, Adnams CM, Seedat S, Parry CDH, Hoyme HE. Who is most affected by prenatal alcohol exposure: Boys or girls? Drug Alcohol Depend. 2017. Aug 1;177:258–267. doi: 10.1016/j.drugalcdep.2017.04.010. Epub 2017 Jun 15. PMID: 28624747 [DOI] [PubMed] [Google Scholar]
- 10.Mage DT, Donner M. Female resistance to hypoxia: does it explain the sex difference in mortality rates? J Womens Health (Larchmt). 2006. Jul-Aug;15(6):786–94. doi: 10.1089/jwh.2006.15.786. PMID: 16910910. [DOI] [PubMed] [Google Scholar]
- 11.Lewis PJ, Boylan P. Alcholo and fetal breathing. The Lancet. 1979: 388. [DOI] [PubMed] [Google Scholar]
- 12.Dubois CJ, Kervern M, Naassila M, Pierrefiche O. Chronic ethanol exposure durign development: Disturbances of breathing and adaptation. Respir. Physiol. Neurobiol 2013, 189(2):250–260. [DOI] [PubMed] [Google Scholar]
- 13.Macchione AF, Trujillo V, Anunziata F, Sahonero M, Anastasia A, Abate P, Molina JC. Early ethanol pre-exposure alters breathing patterns by disruptions in the central respiratory network and serotonergic balance in neonate rats. Behav Brain Res. 2021. Jan 1;396:112908. [DOI] [PubMed] [Google Scholar]