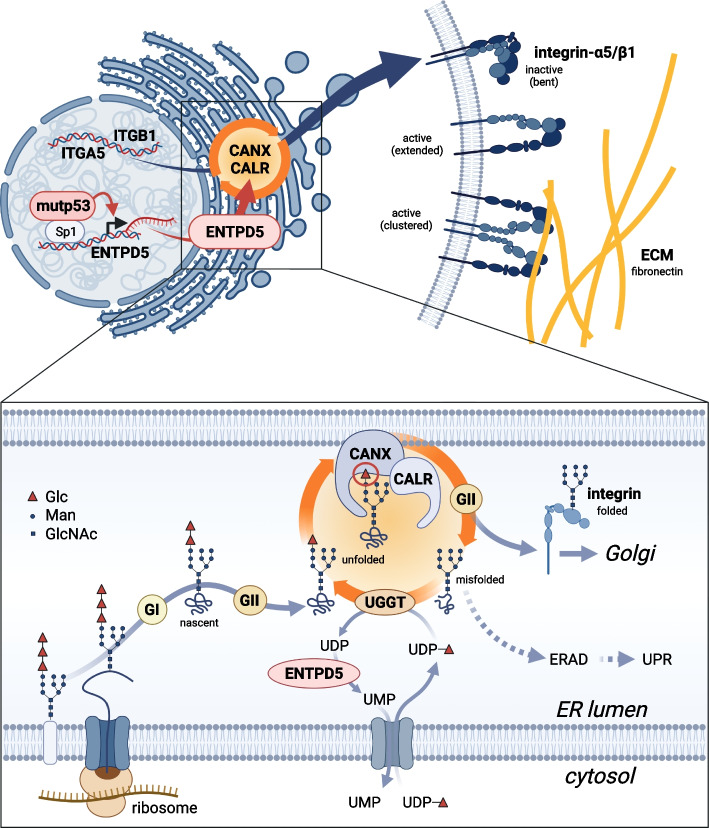
Fig. 8.
Graphical abstract illustrating the mechanism of mutp53-driven integrin-α5/β1 expression. Mutant p53 (mutp53) proteins induce the transcription of ectonucleoside triphosphate diphosphohydrolase 5 (ENTPD5). ENTPD5 operates in the endoplasmic reticulum as a UDPase to generate UMP. Subsequently, UMP is exchanged for UDP-glucose, which is required by UDP-glucose:glycoprotein glucosyltransferase (UGGT) to add a single glucose moiety (represented by a red triangle) to unfolded N-glycoproteins, including integrins. The presence of a single, terminal glucose residue enables the interaction with the lectin chaperones calnexin (CANX) and calreticulin (CALR), promoting proper folding. α-glucosidases I and II (GI, GII) trim glucose moieties and, along with UGGT, regulate the entry and exit of integrins from the CANX/CALR cycle. Once correctly folded, the integrins exit the ER and are transported to the cell membrane through the Golgi apparatus. To avoid the accumulation of misfolded proteins, cells employ the endoplasmic reticulum-associated degradation (ERAD) pathway or activate the unfolded protein response (UPR) mechanisms. At the cell membrane, integrin-α5/β1 dimers are activated, adopting an extended conformation, and form clusters to mediate adhesion to fibronectin, a component of the extracellular matrix (ECM). This process promotes cellular migration, invasion, and metastasis. Created with BioRender.com