Table 1.
Exploration of the reaction conditionsa for the photochemical synthesis of functionalized bicyclo[3.1.1]heptanes.
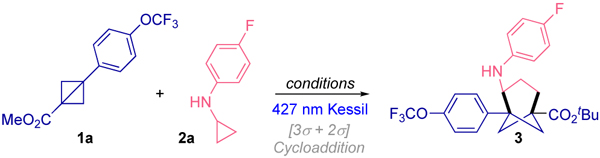
| |||
|---|---|---|---|
| Entry | Solvent | PC | Yield of 3 (%)b |
| 1 | DMSO | Ir[dF(CF3)ppy]2(dtbpy)PF6 | 72 (70)e |
| 2 | DMSO | Ir(ppy)3 | <10 |
| 3 | DMSO | Ru(bpy)3(PF6)2 | 30 |
| 4 | DMSO | MesAcr | traces |
| 5 | DMSO | 4CzIPN | 27 |
| 6 | DMA | Ir[dF(CF3)ppy]2(dtbpy)PF6 | 46 |
| 7 | MeCN | Ir[dF(CF3)ppy]2(dtbpy)PF6 | 40 |
| 8 | 1,4-dioxane | Ir[dF(CF3)ppy]2(dtbpy)PF6 | 0 |
| 9 | MeNO2 | Ir[dF(CF3)ppy]2(dtbpy)PF6 | traces |
| 10 | DMSO | none | 0 |
| 11c | DMSO | Ir[dF(CF3)ppy]2(dtbpy)PF6 | 0 |
| 12d | DMSO | Ir[dF(CF3)ppy]2(dtbpy)PF6 | 0 |
Reaction conditions: 1a (0.1 mmol, 1 equiv), 2a (0.2 mmol, 2 equiv), photocatalyst (2 mol % metal-based PC or 5 mol % organic based PC), in dry and degassed solvent (0.2 M) under blue Kessil irradiation (λmax = 427 nm) at rt.
Yields determined by 1H NMR using 1,3,5-trimethoxybenzene as internal standard.
Reaction in the absence of light irradiation.
Reaction as in entry 5 but in the presence of 5 equiv of TEMPO.
Isolated yield from 0.2 mmol scale.
