Abstract
The neurovascular unit (NVU) reflects the close temporal and spatial link between neurons and blood vessels. However, the understanding of the NVU in the spinal cord is far from clear and largely based on generalized knowledge obtained from the brain. Herein, we review the present knowledge of the NVU and highlight candidate approaches to investigate the NVU, particularly focusing on the spinal cord. Several unique features maintain the highly regulated microenvironment in the NVU. Autoregulation and neurovascular coupling ensure regional blood flow meets the metabolic demand according to the blood supply or local neural activation. The blood–central nervous system barrier partitions the circulating blood from neural parenchyma and facilitates the selective exchange of substances. Furthermore, we discuss spinal cord injury (SCI) as a common injury from the perspective of NVU dysfunction. Hopefully, this review will help expand the understanding of the NVU in the spinal cord and inspire new insights into SCI.
Keywords: Blood–spinal cord barrier, neurovascular coupling, neurovascular unit, spinal cord, spinal cord injury
Introduction
Proper structural and functional homeostasis of the central nervous system (CNS) requires precise regulation of blood perfusion, oxygen delivery, and energy supply.1,2 The CNS has two unique vascular features to maintain homeostasis: the neurovascular unit (NVU) ensures adequate blood supply according to neural activation and metabolic waste removal, and the blood–central nervous system barrier (BCNSB) partitions the circulating blood from the parenchyma to permit the selective exchange of substances. 3 The blood–brain barrier (BBB) and blood–spinal cord barrier (BSCB) have similar barrier abilities and are sometimes referred to as BCNSB. 4 These key features in the NVU are maintained by the highly coordinated activity of multiple cell types.5–7 The NVU has attracted enthusiastic interest in the scientific community for its status in brain function. However, the spinal cord, another essential organ in the CNS, has received less attention. The current understanding of the NVU in the spinal cord typically has to follow the paradigm obtained from the brain.
It has been widely accepted that BCNSB disruption contributes to neurological deficits.2,8,9 Extreme alterations of the NVU are thought to result in tissue ischemia and energy supply deficits, further promoting neurological disorders. 10 Diverse immune cells are strategically distributed within the NVU and associated with neuroinflammatory response in diseases. 11 Thus, studies of the BCNSB and NVU can help in understanding the mechanisms of neurological disorders and may provide novel treatments for these diseases, such as ischemic stroke, traumatic brain injury, and vascular dementia.12–14 However, to date, interpretation of the mechanisms of the NVU and BSCB are insufficient in injured spinal cord. Spinal cord injury (SCI) is a devastating injury that causes long-term disability. The primary insult initiates a sustained impairment cascade called secondary injury, which continues to exacerbate the degree of SCI. 15 Insults to the spinal cord can disrupt the functional or structural integrity of the NVU and BSCB, thus playing a vital role in secondary injury.16,17 Therefore, we will discuss the status of the research on the NVU and BSCB dysfunction in SCI.
The neurovascular unit
It was observed that the blood supply to the CNS was not exclusively controlled by systemic circulation; the brain could regulate its blood supply and metabolic needs in active brain regions by neural activity. The concept of the NVU was proposed to study the pathological mechanisms of stroke by the National Institute of Neurological Disorders and Stroke Progress Review Group in 2001, emphasizing the unique relationship between brain cells and cerebral vasculature. 10 This concept emerged as a new paradigm for investigating physiology and pathology in the CNS.
Today’s view of the NVU has been broadly amplified. BCNSB is currently preferred as the main functional configuration of the NVU. 1 In addition to its barrier function which preserves the normal function of the CNS, the BCNSB also actively serves as a communication interface for the NVU between the periphery and the CNS. In contrast, interactions in the NVU are critical for the formation and maintenance of the BCNSB.5,10 The role of the NVU in regulating cerebral blood flow has been broadly categorized as (1) autoregulation, the response of the cerebral vasculature to changes in perfusion pressure;18,19 (2) neurovascular coupling, the local blood flow response to changes in neural activity;3,20 (3) vascular reactivity to metabolic stimuli, such as pH or CO2 content; 21 and (4) endothelium-dependent responses, such as hemodynamic stimuli (e.g., shear stress), neurotransmitters, and pharmacological agents. 22 Nevertheless, some functions of the NVU are difficult to distinguish clearly in experiments and therefore are often misidentified in studies.
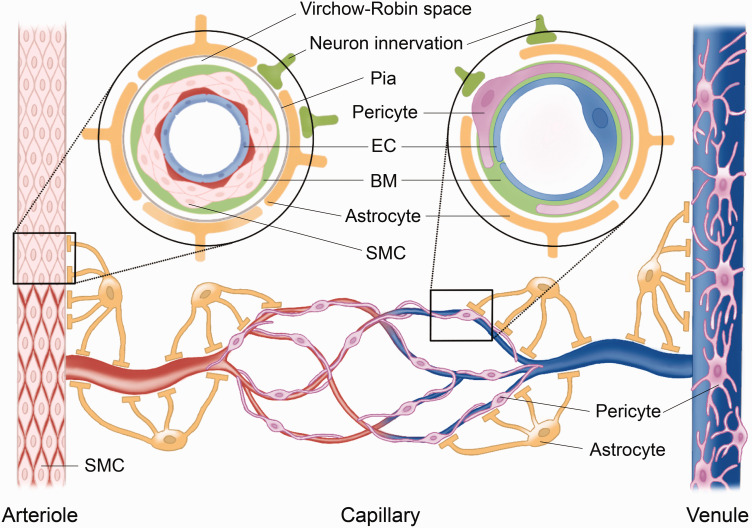
At different levels of the vascular network, diverse types of cells and associated structures play diverse roles in cerebrovascular function and homeostasis synergistically throughout the CNS. 23 Collectively, the BCNSB comprises endothelial cells (ECs), basement membrane (BM), pericytes, smooth muscle cells (SMCs), and glia limitans. Interactions among these different vascular cells with neurons and astrocytes refine the functions of the BCNSB and act as a whole NVU (Figure 1). 24 In the following section, we discuss the common features of cells in the NVU, supplemented by available studies from the perspective of the brain.
Figure 1.
The typical components of the neurovascular unit (NVU) in the central nervous system (CNS). The NVU comprises (1) vascular cells, including endothelial cells (ECs) and mural cells, such as pericytes on capillaries and venules, or smooth muscle cells (SMCs) on arterioles and small arteries; (2) neuroglial cells, such as astrocytes; and (3) neurons. ECs form the luminal layer of the vessel wall. The space between adjacent ECs is held by paracellular junctions. The expression of some junctional proteins (including claudin-11, ZO-1, occludin, catenin, and VE-cadherin) is lower in the BSCB than in the BBB. At the arteriolar level (left inset), the basement membrane (BM) and SMC envelope endothelium. Astrocytic endfeet insert into the BM to regulate SMCs through the pia and the glia limitans. The capillary and venule (right inset) lack SMCs, where pericytes embed in the BM and wrap around the ECs on the abluminal side. The astrocytic endfeet attach to the pericytes at both venules and capillaries. Pericyte coverage is less on the BSCB than on the BBB. SMCs, pericytes, and astrocytes all receive neuronal innervation to control their function. In addition, these conformations of cells are also the major cellular structure of the blood–central nervous system barrier.
Neurons
Neurons are protected by the BCNSB to ensure normal function. 2 Neurons can regulate nearby blood vessels by generating signals directly or via interposed cells based on their oxygen or metabolic demands. Neuron terminals can trigger calcium signaling in astrocyte processes via several mechanisms, thus preceding the onset of vascular responses. 25 Classically, neurons release glutamate to activate the ionotropic and metabotropic glutamate receptors on astrocytes, evoking Ca2+ release from internal stores and increasing the intracellular Ca2+, leading to activation of downstream Ca2+-dependent enzymes and generation of vasodilatory substances. 26 Thus, astrocytes take over to adjust the blood supply by modulating local vascular cells.
Moreover, studies have shown that neurons release various neurotransmitters directly or indirectly via astrocytes and pericytes to mediate vascular changes in the brain. 27 Diverse signaling cascades may involve this process at the capillary or arteriole levels. 26 Neuronal activity-induced capillary dilation classically relies on glutamate receptor (mainly metabotropic) activated Ca2+-dependent signaling in astrocytes. In contrast, neurovascular coupling-evoked arteriole dilation in the brain depends on NMDA receptor activation and Ca2+-dependent NO generation by interneurons. 26 Different types of interneurons have been suggested to control local blood flow responses differently. 27 More information is needed to identify the exact function of these neurotransmitter signals in the NVU at the level of the spinal cord.
Astrocytes
Small arteries penetrating the neural parenchyma are completely enveloped by astrocytic endfeet, which occupy a larger surface area of the microvasculature than the neural processes. 28 Astrocytes also ensheath capillaries to constitute the most abluminal layer in the NVU. 29 In the human cortex, a single astrocyte might sense the activity of more than one million neuronal synapses in its domain by the fine endfeet. 30 Moreover, each astrocyte has at least one process with its endfeet surrounding the cerebral blood vessel. 31 Characterized by highly ramified processes contacting the neural processes and microvessels, astrocytes are well positioned to link neural activity to microvascular function. 32 Astrocytes are expected to modulate local perfusion by neuron-to-astrocyte signaling. 33 In vivo imaging has verified a frequency-related correlation between neuronal activity, Ca2+ transients in astrocytes, and vasomotility. 34 Astrocytic Ca2+ transients are less synchronized with low-frequency neuronal Ca2+ transients and do not seem to contribute to low-frequency hemodynamic changes. However, under high-frequency-stimulated neuronal Ca2+ responses, astrocytic Ca2+ transients are cumulative and temporarily correlated with vasoconstriction. 35 In the CNS, astrocytes can detect changes in both blood and neurons through several neurotransmitters, mainly glutamate and γ-aminobutyric acid, and evoke downstream calcium signaling. 36 These calcium signals can activate smooth muscle cells via potassium signaling and the Na+-K+ ATPase, 37 causing local vasodilation and then propagating away to induce vasomotion in adjacent arterioles.32,38 Moreover, astrocytes also produce and release various molecular mediators, such as prostaglandins, nitric oxide (NO), and arachidonic acid, to regulate local blood flow in a coordinated manner.39,40
Astrocytes alone are not to determine barrier properties because astrocytes appear postnatally in the brain, well after the BBB has already been sealed. 41 Nevertheless, astrocytes still contribute to BBB properties by releasing molecules to promote BBB repair, restrict peripheral immune cells, and assist in the resolution of inflammation. 42 Astrocyte-derived factor induces endothelial polarization and produces glycocalyx on the CNS endothelium. 29 Astrocyte-derived Sonic hedgehog signaling stimulates the expression of tight junction (TJ) proteins such as claudin-5 and occludin in the CNS endothelium. 43 In addition, astrocytes can also generate and release vascular endothelial growth factor, which downregulates claudin-5 and occludin through activation of endothelial nitric oxide synthase (NOS) and increases BBB permeability. 44
Endothelial cells and smooth muscle cells
The ECs lining the lumen of vessels are the core anatomical unit of the barrier directly facing the blood flow, regulating transportation and communication between the CNS and blood circulation. Many features of ECs contribute to the barrier property. The paracellular junctions, mainly including tight junctions (TJs) and adherens junctions, hold the space between adjacent ECs and limit the paracellular flux of solutes. 45 These paracellular junctions primarily confer low paracellular permeability and high electrical resistance to the barrier in the CNS.6,46 The majority of the regulated transportation occurs through the paracellular route. 47 In addition, the ECs in BCNSB offer low-rate vesicle-mediated transportation across the endothelium, called transcytosis.45,48 This form of transportation is designed to transport macromolecules mediated by specialized structures, including clathrin, caveolae, and vesiculo-vacuolar-organelles. 49 Vesicles are likely to be a highly efficient and economical way of exchanging information across the BBB. 50 However, transcytosis is understudied in the spinal cord.
ECs and SMCs are major vasomotion effectors in the NVU that regulate blood flow in response to signals from neurons and astrocytes. ECs are equipped with a repertoire of voltage-gated ion channels, ligand-gated ion channels, and G-protein-coupled receptors, which provide the ability to respond to multiple physiological inputs in the CNS.3,51 Diverse bioactive molecules can elicit endothelial functions to regulate hemodynamics in the brain, and many of them are autocrine, such as adrenomedullin, angiotensin, NO, prostanoids, and several others. 52 ECs also propagate vasomotor responses to regulate blood flow in the CNS in response to both chemical and mechanical signals from the luminal side. 53 Shear stress evoked by flowing blood and impinging on the endothelial surface can induce the release of dilatory NO. 53 Therefore, there is likely a positive feedback mechanism in which dilatation increases the blood flow at arterioles; thus, increased shear stress and release of NO can cause further vascular dilatation downstream. 54 The mechanosensory effect of shear force is probably mediated by the endothelial glycocalyx with changing oncotic pressure gradients at the endothelium surface, 55 transmitted by the pathway platelet endothelial cell adhesion molecule-1, vascular endothelial cell cadherin, and PI3 kinase. 56 Alternatively, the mechanosensory effect may be directly transduced by mechanosensitive ion channels, such as inwardly rectifying potassium channels (KIRs) and transient receptor potential cation channels V4 and Piezo-1. 57
The primary function of SMCs is to maintain the vascular tone or narrow vessel diameter to increase intravascular pressure, 58 by receiving signals from ECs, tissue metabolism, humoral stimuli, and neural stimuli. 59 ECs can directly affect SMCs via several mechanisms, including the release of signaling molecules such as NO, or electrical signals via myoendothelial gap junctions between ECs and SMCs. 60 In addition to directly responding to neurotransmitters released by neurons, the SMC also responds to the modulated signaling within astrocytes in the CNS. Neuronal activity can promote a calcium signal in astrocytic endfeet decoded by Ca2+-sensitive K+ channels, which locally release K+ into the perivascular space to activate SMC inward rectifier K+ channels and cause vasodilation. 37 ATP from both neurons and astrocytes can activate purinergic receptors on SMCs. The activation of purinergic receptors P2X and P2Y in SMCs has been shown to cause an increase in Ca2+, thus causing SMC contraction. 61 The influx of Ca2+ across the plasmalemma or the release of Ca2+ stores from the sarcoplasmic reticulum in SMCs could activate contractile proteins and narrow the vascular diameter. 58 In capillaries and postcapillary venules that lack SMCs, pericytes are thought to be the major regulators that replace SMCs. 62 However, vessel dilation in capillaries occurs seconds prior to arterioles mediated by neurovascular coupling in the cerebral cortex. 34 Whether there is a functional relationship between SMCs and pericytes remains unclear. Furthermore, the contraction and relaxation of SMCs are simultaneously governed by multiple mechanisms from different cells in the NVU, which appears redundant and inconvenient, yet the physiological significance of this type of overlapping control on SMCs is not well understood.
Pericytes
Pericytes are embedded in the basement membrane at the capillary abluminal side. 29 Several neurovascular functions necessary for CNS homeostasis are regulated by pericytes, including BCNSB maintenance, vascular angioarchitecture, vesicle trafficking, and neurovascular coupling.63,64 Recruitment of pericytes to the capillary wall is a critical marker for maturity of the BCNSB. 5 The pericyte synthesizes many elements of the basement membrane, such as proteoglycans and laminal proteins, which are thought to be critical in the differentiation of BCNSB. 65 Unlike astrocyte endfeet, pericyte remodeling is likely nonexistent in the brain. 66 This property may be essential in restoring capillary flow in injury or aging but has not been verified in the spinal cord. Pericytes regulate the function of BCNSB in at least two ways: regulating BCNSB-specific gene expression in ECs and inducing polarization of astrocyte endfeet surrounding blood vessels. 67 Pericytes probably control the expression of endothelial paracellular junctions, the alignment of TJs, and bulk flow transcytosis of vesicles across the BCNSB. 68 Coculture of pericytes with ECs has shown enhanced barrier function with increased expression of TJs and decreased permeability under healthy and hypoxic conditions. 69 Pericyte-deficient mouse models have increased permeability of the BBB,64,67 and reduced TJ proteins expression. 70
Pericytes are major regulators that adjust blood flow in the capillaries and postcapillary venules, 62 by vasomotion according to neuronal activity changes in the CNS. 34 Pericytes possess contractile proteins, including the mesenchymal intermediate filament proteins vimentin and microfilaments that are assembled to actin and myosin, and to the contraction-related proteins tropomyosin and desmin. 71 Pericytes have a Ca2+-dependent contraction in response to neurotransmitters, neuronal activity, or electrical stimulation in neurovascular coupling.27,72 Neurotransmitters such as norepinephrine cause pericyte contraction, while gamma-aminobutyric acid, adenosine, glutamate, and dopamine cause pericyte relaxation. 68 These signals activate purinergic receptors or voltage-gated Ca2+ channels expressed on pericytes to increase intracellular Ca2+ and cause pericyte contraction.68,73 Activating K+ channels decreases Ca2+ influx and causes pericyte relaxation in response to high lactate and carbon dioxide levels.27,40,74 Moreover, capillary dilation is reduced by blocking NOS, suggesting a role for NO in pericyte-induced capillary dilation and contraction. 62 Such signals in neurovascular coupling for contraction can propagate between pericytes in the brain, probably transmitting through gap junctions between pericytes or ECs. 40 Hence, it has been theorized that the vascular responses may be initiated by pericytes in capillaries and then propagated to upstream arterioles, because pericytes are closer to the active neurons than arterioles in the brain, and the neuronal activity activates an outward membrane current in pericytes that dilate capillaries before the arterioles.40,62
In pericyte-deficient mice with decreased pericyte coverage, cerebral microcirculation perfusion, oxygenation levels, and neurovascular coupling are diminished, 72 and the BBB is dysfunctional resulting in an accumulation of neurotoxic substances in the brain. 70 Physiologically, the number of pericytes and their capillary coverage in the spinal cord are lower than those in the brain, 75 probably indicating that the regulating ability of capillaries is weaker in the spinal cord. In chronic SCI, the paradoxical excess activity of monoamine receptors on pericytes causes local capillaries to constrict abnormally and reduce blood flow. 76
Basement membrane
The BM is an essential accessory structure to seal the BCNSB, provide structural support and allow intercellular signaling communication in the NVU. 29 The space of discontinuous pericyte coverage and the space between astrocytes and endothelium are padded by BM. The BM is an extracellular matrix that in itself does not prevent small molecules from entering the CNS. Instead, it probably serves to restrict the passage of immune cells and provides a specific perivascular microenvironment for substance exchange and signaling communication. 77 Disruption of the BM can lead to alterations in the cytoskeleton of the endothelium, in turn affecting TJ proteins and barrier integrity. 65
The BM in the CNS is mainly comprised of type IV collagen, fibronectin, laminins, and other glycoproteins. Collagen IV and fibronectin are secreted by multiple cells in the NVU, while deletion of either one is embryonic lethal. 78 Laminin contributes to BCNSB regulation and pericyte differentiation, 29 in addition to repairing BBB after ischemic brain injury. 79 Conditional deficiency of laminin leads to compromised BBB integrity with decreased pericyte coverage, diminished aquaporin-4 and TJs expression, and enhanced transcytosis. 80 Two main families of ECM receptors predominate in the BM, including dystroglycans and integrins. Laminin α2–dystroglycan receptor interactions regulate the maturation and function of the BBB. 81 Integrin β1 is indispensable for proper VE-cadherin signaling and paracellular junction organization in the BBB. 82 The degradation of the BM opens a conduit that enhances the migration of inflammatory cells into the parenchyma to aggravate the secondary injury. 83
Multiple proteases regulate the extracellular matrix (ECM) in the BM, such as matrix metalloproteinases (MMPs). 84 Currently, twenty-four human MMP homologs have been categorized into six subfamilies: collagenases, gelatinases, stromelysins, matrilysins, membrane-type metalloproteinases, and others. 85 MMPs are typically maintained at low expression levels in the adult CNS, which is necessary for maintaining normal functions of the ECM and is modified in pathological conditions.85,86 Some MMPs, such as MMP-1, -2, -3, -7, and -9, have been found to upregulate and break the BM in CNS injury. 87 These MMPs cleave the ECM, diminish signaling in the ECM, and degrade the paracellular junction proteins, thus disrupting BCNSB. 88 MMP-2 and MMP-9 can cleave dystroglycan to break the anchors of astrocyte endfeet. 89 MMP-8 and MMP-13 are also reported to be upregulated during neuroinflammation and are associated with BBB damage. 90 In addition, increased MMP-12 regulates ECM remodeling by inactivating some proteases, which is correlated with the development and myelination of the CNS. 84 The upregulation of MMPs has been widely reported to deteriorate the BSCB in SCI,9,16 mainly including MMP-2, -3, and -9. 91 MMP-9 is the main contributor to disrupting the BSCB after SCI because MMP-9 inhibition or deficiency can reduce barrier disruption. 92 However, MMP-2 and MMP-9 likely support tissue repair by ECM remodeling to facilitate angiogenesis and glial scar formation in chronic SCI. 93 MMP-2 deficiency leads to vascular instability, a decline in vascularity, and prolonged MMP-9 upregulation in SCI. 94 Moreover, MMP-12 knockout mice showed reduced permeability of the BSCB and improved functional recovery after SCI. 92
Other cells
Oligodendrocytes and microglia constantly reside around the NVU but are not traditionally considered part of it. The role of oligodendrocytes or oligodendrocyte precursor cells in the NVU is partially conclusive. Oligodendrocytes ensheath axons and are adjacent to astrocytes, pericytes, and endothelial cells in cerebral white matter. 95 The possible role of oligodendrocytes in the NVU may be to engage in cerebral vascular remodeling or regeneration through Wnt or Nogo signaling and release matrix metalloproteinase. 96 Perivascular microglia are immune cells in the CNS that inhabit the NVU and respond to changes in the NVU in disease. These microglia probably have dual-directional effects on the NVU, protecting NVU integrity in acute injury but engulfing astrocytic end-feet and increasing barrier permeability in chronic injury. 97 In addition, microglia probably have an unrecognized role in modulating blood flow and neurovascular coupling in the brain through P2RY12 signaling to interact with other cellular components in the NVU.98,99
Autoregulation
Autoregulation is a self-adjusted mechanism involving constriction or dilation of cerebral resistance vessels to maintain constant tissue perfusion within a range of systemic mean arterial blood pressure (MAP). The vessels in the CNS constrict autonomically to impede blood flow when MAP increases, or dilate to facilitate blood flow when MAP decreases or the partial pressure of CO2 increases. 23 This buffering system in the CNS is designed to stabilize blood perfusion to match metabolic need and maintain neural function during systemic blood pressure fluctuations. Lassen et al. first proposed this concept and described a plateau region wherein cerebral blood flow remains relatively stable across a range of MAP (60–150 mmHg). 100 Interestingly, Birch et al. identified that the relative capacity to buffer changes in cerebral blood flow is strongly dependent on the speed of changes in MAP. The slower the change in MAP, the smaller the impact on cerebral blood flow, to a point where cerebral blood flow becomes almost unaffected. 101 Autoregulation ability of the spinal cord mirrors that of the brain, providing a similar mechanism for maintaining balance for spinal cord blood flow (SCBF). 102
However, the mechanism of autoregulation in the spinal cord seems more complicated. The autoregulation is still preserved after operationally sacrificing the segmental arteries of the spinal cord. 103 Autoregulation remains intact in the thoracic spinal cord after the high cervical spinal cord is completely severed. 104 SCBF and local field potentials are not significantly affected and remain coupled in the preserved spinal cord segments, indicating that spinal cord autoregulation allows a potent adaptation when significant fluctuation in MAP occurs or supraspinal control is lost. 105
Autoregulation in the CNS is now divided into two categories: “static” and “dynamic” autoregulation.18,21 The former refers to blood perfusion or blood flow in the CNS maintaining a relatively steady state when MAP varies under physiological conditions. 106 Dynamic autoregulation refers to the ability of autoregulation to respond to rapid changes in blood pressure from seconds to minutes. The effectiveness of static autoregulation can be even more prominent with lower frequency changes in MAP. The progressively slower changes in MAP result in more minor changes in cerebral blood flow. 18 Therefore, the MAP and cerebral blood perfusion are usually measured and averaged over longer time intervals (10 min or more) in experiments to make the resulting values adequately represent static autoregulation. 18 In contrast, dynamic autoregulation requires high temporal resolution techniques to measure the fast changes in blood flow. 107 During more rapid changes in MAP, there is much greater variability in cerebral blood flow than the assumption based on Lassen’s concept of static autoregulation. 18
The relationship between static and dynamic autoregulation has not been clearly identified. Tiecks et al. reported a robust linear relationship between static and dynamic autoregulation in the brain, probably denoting that static autoregulation is additionally maintained based on the constant and rapid adjustment of dynamic autoregulation. 108 Otherwise, they are opposite types of adaptive responses. As de Jong et al. suggested, there is a weak relationship between static and dynamic autoregulation in the brain, which has significant variability between individuals and sex. 109 In addition, after high-level SCI, static autoregulation is well maintained, while dynamic cerebral autoregulation is markedly altered, supporting the view that static and dynamic autoregulation are separated. 110
Anatomical basis of spinal cord autoregulation
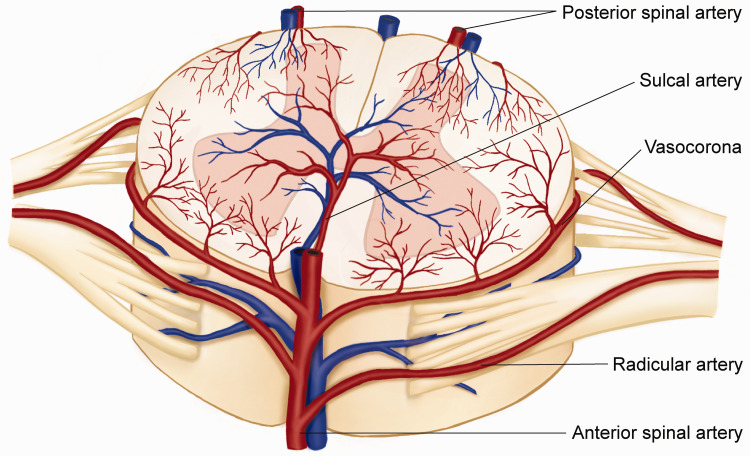
In humans, the parenchyma of the spinal cord is supplied by branches of several major arteries, including the vertebral and posterior-cerebellar arteries. The anterior radicular artery enters the spinal cord bilaterally, with each anterior root joining the anterior spinal artery, which descends on the ventral surface of the spinal cord. Small branches of the vertebral or posterior-inferior cerebellar arteries continue caudally over the dorsal spinal cord, usually forming two small trunks known as the posterior spinal arteries (Figure 2). While the venous drainage of the spinal cord may be variable, its anatomic pattern often runs parallel to the paired artery.
Figure 2.
A dorsal view of the vascular anatomy of the spinal cord. The spinal cord is supplied by a net-like anastomosing vascular system from the branches of longitudinal anastomotic trunks: one anterior spinal artery and two posterolateral spinal arteries. The pattern of venous return is often parallel to the anatomic arteries. The spinal arteries are supplied by the aorta and lack the siphon structure as in the brain to attenuate cardiac pulsatile blood flow variation. The blood flow direction can change in the median spinal vessels and vasocoronas due to multiple traffic branches. Theoretically, there is a dead point where the opposing pressures stop the blood flow. The location of the dead point is constantly changing due to physical activity or blood pressure fluctuation, indicating that autoregulation in the spinal cord is more sophisticated than in the brain.
Cardiac pulsatile flow can cause fluctuating hemodynamic changes in the arterial vessels of the spinal cord. Moreover, the vessels entering the spinal cord lack the siphon structure seen in the brain (such as the internal carotid artery siphon) to attenuate pulsatile variation of blood velocity and pressure. 111 How autoregulation of the spinal cord adjusts to these pulsatile hemodynamic changes is unclear. Theoretically, the pulsatile pressure load from blood flow in the spinal cord is supposed to be alleviated by large elastic arteries, as the arteries distend when the blood pressure rises during systole and recoil when the blood pressure falls during diastole, called Windkessel effect. 112 There is also a novel hypothesis that an extensive array of highly innervated arteriovenous glomeruli found in the spinal cord might be involved in the autoregulation of SCBF, because these arteriovenous anastomoses are similar in the pads of mammalian extremities or penis to bypass the capillaries, 113 but more data are needed to prove this hypothesis.
Signal transmission in autoregulation
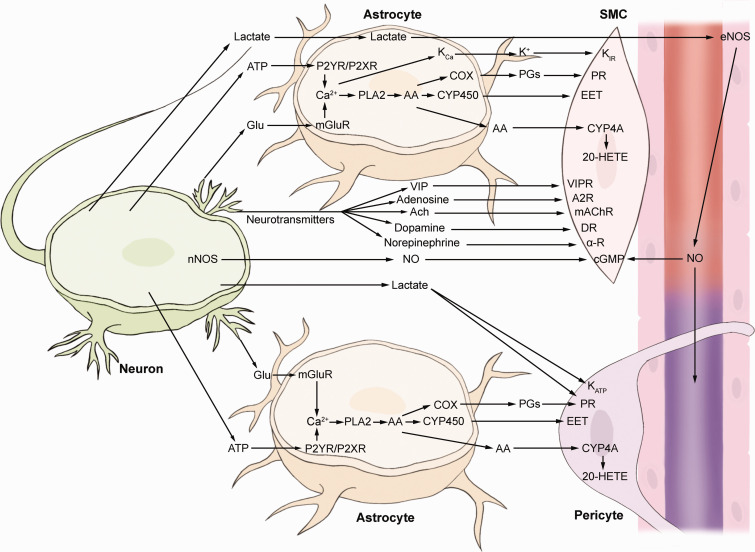
Autoregulation in the CNS is considered to operate through multiple mechanisms including myogenic, metabolic, neurotransmitter-mediated, neurogenic, and systemic control (Figure 3).20,51 The myogenic mechanism encompasses the direct reflex response of vessels to variations in mechanical force caused by blood flow, such as perfusion pressure, shear force, and stretch force, which dictate the responses of ECs and SMCs. 51 The metabolic mechanism of blood flow control in the CNS involves metabolic byproducts, such as lactate or CO2, to regulate blood perfusion. 51 Accumulating extracellular lactate can induce vessel relaxation which adjusts to the metabolic needs of neurons. 114 Gap junction-coupled astrocytes can remove lactate from the extracellular fluid and discharge it through their endfeet into the perivascular fluid. 115 The released lactate then causes vasodilation by some different mechanisms, either by releasing endothelial vasoactive substances such as NO and hindering the transport of extracellular prostaglandin,27,40 or by interfering with the metabolism of surrounding cells via the lactate/pyruvate ratio to release vasoactive substances.74,115
Figure 3.
Simplified schematic diagram of the major pathways that regulate blood flow in the cerebral NVU. Neuronal presynaptic release of glutamate (Glu) is taken up by astrocytic processes and acts on metabotropic glutamate receptors (mGluR) to raise [Ca2+], inducing the generation of arachidonic acid (AA) from phospholipase A2 (PLA2). The ionotropic glutamate receptors seem less important in controlling blood flow in the spinal cord, and this signaling is not shown in the figure. AA can be converted to multiple prostaglandins (PGs) (by cyclooxygenase, COX), epoxyeicosatrienoic acid (EET) (by cytochrome P450 epoxygenase, CYP450) to dilate vessels, or 20-hydroxyeicosatetraenoic acid (20-HETE) (by ω-hydroxylase, CYP4A) to constrict vessels. Raised [Ca2+] in astrocyte endfeet may activate Ca2+-gated K+ channels (KCa) to release K+ and activate inward rectifier K+ channels (KIRs) on SMCs. The metabolic process of neurons releases adenosine triphosphate (ATP) acting on metabotropic purinergic receptors (P2XR or P2YR) on astrocytes to increase intracellular Ca2+. The metabolic byproducts adenosine (to adenosine 2 receptor, A2R) and lactate activate ATP-sensitive potassium (KATP) channels, causing vessel relaxation; lactate inhibits PG transporters to cause PG accumulation or activate endothelial nitric oxide synthase (NOS) to release nitric oxide (NO), subsequently causing vasodilation. Neurons release diverse vasoactive neurotransmitters to control the NVU directly, such as vasoactive intestinal peptide (VIP) (to VIP receptor, VIPR), acetylcholine (ACh) (to muscarinic acetylcholine receptor, mAChR), dopamine (to dopamine receptor, DR), norepinephrine (to α-adrenoceptor, α-R), and NO (to cyclic guanosine monophosphate, cGMP).
The vasculature in the CNS is known to be innervated by (1) sympathetic nerves, (2) parasympathetic nerves, and (3) sensory nerves. 116 Sympathetic control plays a vital role in the neurogenic autoregulation of the spinal cord. Autoregulation can be eliminated in animals receiving paravertebral sympathectomies, causing the SCBF to vary in a linear relationship with the changes in MAP. 117 The neurotransmitter theory of autoregulation is very complicated. Neurons can release multiple neurotransmitters from nerve endings via the nervi-vasorum, 51 or hierarchically through astrocytes and pericytes,32,118 to adjust vascular reactivity to control vascular reactivity. 119 Several independent studies have reported that many neurotransmitters are involved in autoregulation (Figure 3). Some neurotransmitters are directly vasoactive, such as adenosine, acetylcholine, catecholamines, and neuropeptides; 37 other neurotransmitters are not vasoactive but stimulate the production of vasodilators, including NO, metabolites of cyclooxygenase-2 (COX-2) and P450 epoxygenases, through downstream enzymatic activation in astrocytes and vascular cells.33,119
However, it is difficult to discern the independent autoregulation mechanism in the CNS through inhibition or activation in experimental investigations because overlapping effects in the NVU provide redundant or synergistic effects in autoregulation. 20 Moreover, despite their sound theoretical background, these signaling molecules have broad communication and sometimes are able to activate each other. Thus, no single method has been universally accepted as a gold standard. 19 In addition, side effects may interfere with results in different pharmacological experiments, 21 easily disrupting the precise balance and confusing the understanding of this autocontrol mechanism in the NVU.
Neurovascular coupling
One vital role in the prototypical functions of the NVU is neurovascular coupling, previously called functional hyperemia. 10 neurovascular coupling refers to the link between neural activity and blood flow, resulting in a rapid dilatation of blood vessels and blood flow acceleration near the activated neurons with high spatial and temporal correspondence.3,20,23 In animal models, focal spinal cord perfusion was observed to increase markedly during peripheral nerve stimulation. 120 These results suggest that neurovascular coupling in response to increased metabolic demands of neurons also exists in the spinal cord.
Neurovascular coupling is mainly regulated by neuron-astrocytic interactions in the NVU. Neurons release glutamate to activate metabotropic glutamate receptors on astrocytes and release Ca2+, leading to the activation of downstream Ca2+-dependent enzymes and the generation of vasodilatory substances. 26 In addition, ionotropic NMDA glutamatergic receptors activate NOS and release NO to regulate blood flow. 23 However, pharmacologically inhibiting NMDA receptors does not significantly affect blood flow in the spinal cord, indicating that these signals are not predominant in the spinal cord. 121 Moreover, neurovascular coupling probably induces a remote response transmitted upstream where multiple cells release mediators to engage signaling pathways and activate effectors across the NVU network in a highly orchestrated manner.10,23 These signaling pathways in neurovascular coupling probably involve a feed-forward mechanism, resulting in the release of vasoactive substances, such as extracellular K+, NO, and prostanoids in the brain,34,40 but there is still not enough data to support its existence.10,20 There could also be a relationship between neurovascular coupling and dynamic autoregulation. 122 The activity-induced retrograde propagation of vasodilation may also involve the dynamic autoregulation of the intravascular pressure changes induced by downstream vasodilatation in the brain, 23 but more data are needed to support this possibility.
The data regarding neurovascular coupling in the spinal cord are insufficient. Some reports have shown that task-related or peripheral nerve electrical stimulation can induce a noticeable increase in SCBF. 123 Interestingly, SCBF monitored by fMRI still remains coupled with local field potentials after spinal transection to abolish the changes in MAP. 105 Some BOLD-fMRI reports have detected neuronal activity-related blood flow changes in the spinal cord using averaged values per unit time or the flow-rephasing compensation method to eliminate the influence of pulsatile variation in blood flow. 124 However, these methods may not be suitable for detecting rapid blood flow changes, such as those in functional neurovascular coupling, because these averaged values may reflect the changes caused by dynamic autoregulation.
In addition, various methods quantifying the efficacy of NVU mechanisms accompany their respective limitations, contributing to results that sometimes appear conflicting. 18 New imaging methods have recently provided in vivo evidence that neurovascular coupling exists in the spinal cord. Functional ultrasound imaging detects hemodynamic changes responding to electrical epidural stimulation that reflect neurovascular coupling in the spinal cord. 120 Intrinsic optical signals and light reflectance spectroscopy reveal that the light scattering of vessels and hemoglobin oxygen saturation in the lumbar spinal cord change simultaneously with peripheral electrical stimulation. 125 These results confirm that neurovascular coupling exists in the spinal cord.
Blood–central nervous system barrier
The modern concept of BCNSB was described by Davson et al. as limiting the entrance of plasma components, red blood cells, leukocytes, and foreign pathogens into the neural parenchyma in healthy conditions. 126 Moreover, the CNS parenchyma is thought to lack a potent innate immune response related to this barrier function, termed “immune privilege”. Pathogens introduced directly into the CNS parenchyma evade systemic immunological recognition. 127 Local cell death in the CNS parenchyma does not elicit a stereotypic response of myelomonocytic cells. 128 Despite some controversy regarding whether CNS parenchyma lacks humoral immunity surveillance, 129 the immune privilege or immunologically unique property of the CNS is primarily due to the BCNSB. 128
More functions have been subsequently found in the BCNSB. The BCNSB responds to stimuli that arise within both the CNS parenchyma and blood compartments. 130 In addition, the BCNSB serves as a key homeostatic site for the NVU to regulate nervous tissue perfusion,2,3,5 and a relay station for immune signaling between the blood and CNS via cytokines. 24 Furthermore, the BCNSB acts as both a secretory and endocrine target tissue with endocrine-like properties to enable substance exchange control and communication. 131 The BCNSB releases hormones and neuropeptides into the blood or the interstitial fluid, 132 and is also a target for some hormones, such as leptin and insulin, to regulate glucose metabolism. 133
The endothelium in the CNS lacks membrane fenestrations and pinocytic vesicles (transcellular pathway) when compared to peripheral organs and has extensive paracellular junctions to limit the flux between ECs (paracellular pathway). 65 The paracellular pathway is occupied by paracellular junctions, including TJs, to regulate barrier permeability and provide mechanical stability.2,6,45 The majority of trans-endothelium transport occurs through the paracellular route. 47 TJs are comprised of the claudin family, zonula occludens (ZO) family, TJ-associated MARVEL domain-containing proteins (TAMPs), and junctional adhesion molecules (JAMs).6,46 Claudins comprise TJ strands and play pivotal roles in regulating paracellular permeability. 134 Claudins interact with ZO proteins, which are scaffolding proteins with a cytoplasmic region that anchor to the cytomembrane and are essential for TJ assembly. 6 In addition to TAMPs (including occludin and tricellulin), other members of the immunoglobulin superfamily, such as JAMs, endothelial cell-selective adhesion molecule (ESAM), and nectins, are also associated with TJs. 130 Adherens junctions involve the cadherin family and platelet endothelial cell adhesion molecules. 8 These endothelial junctions impose a high trans-endothelial resistance of 1500–6000 Ω/cm2 in cerebral capillaries and 20–50 Ω/cm2 in the placental barrier. 135 In addition, the barrier has a negative surface charge to repulse anionically charged compounds, 65 which mainly come from the glycocalyx, a negatively charged dense layer of carbohydrates on the luminal side, acting as the first line of barrier and preventing immune cell entry.29,136 Some early studies utilized the loss of anionic charge to reflect BSCB disruption in SCI. 137 However, the disruption of the BCNSB still lacks a direct detection method. 138 In many studies, the common method used to evaluate BCNSB function is to measure the entry of serum proteins or intravenously injected tracers into the CNS. 130
The BCNSB contains multiple substrate-specific transport systems that control the transport of nutrients, energy metabolites, and other essential molecules transported between the interstitial fluid in the CNS and the blood, 5 through passive diffusion, facilitated diffusion, and active transport. 24 The forms of exchanges interact broadly with hemodynamic changes and NVU functions. 47 The intravascular pressure gradient between arterioles and venules is the primary regulator of luminal flow. 135 Dilation of upstream resistance arterioles increases the pressure gradient, thus increasing blood flow in the capillary, which is critical for regulating transportation across the barrier. 135 The physiological shear force corresponding to the increased blood flow in capillaries maintains the barrier phenotype through regulation of junctional protein and substance transportation. 135 BCNSB dysfunction-related blood flow reductions can further contribute to neurological deficits. 2
Comparison of the BSCB and BBB
Traditionally, the characteristics of the BSCB are believed to be similar to the BBB,4,139 despite several minor structural and functional differences. 17 The number of pericytes covering the BSCB is less than that covering the BBB. 75 The large superficial vessels in the spinal cord contain more glycogen deposits, which are not generally seen in the brain. 140 Compared to the BBB, the permeability of the BSCB is considered to be higher in the physiological state because the spinal cord generally takes up more tracers than the brain. 141 In multiple sclerosis or experimental allergic encephalomyelitis affecting both the brain and spinal cord, more blood-derived interferons and tumor necrosis factors reach the spinal cord. 139 Compared to conditioned brain injury, the duration of the BSCB remains highly permeable after conditioned SCI, and the area of BSCB leakage is 2–3 times greater than that of BBB leakage. 139 These differences between the BBB and BSCB may be due to lower expression of TJ proteins (claudin-11, ZO-1, and occludin), adherens junction-associated proteins (beta-catenin and VE-cadherin), or efflux transporters (such as P-glycoprotein) in the spinal cord vasculature compared to the brain.142,143
Advanced approaches to study the NVU in the spinal cord
The first quantitative study to calculate blood perfusion in the spinal cord with an autoradiographic technique by computing the intracardiac injected microspheres lodging in the spinal cord in proportion to the cardiac output. 144 Similar measurements using fluorescent or radioactive microspheres have reported the autoregulation ability in the spinal cord. 145 Later, various methods were developed to measure the SCBF, such as the hydrogen electrode technique, radioactivity or fluorometric angiography technique, Doppler flowmetry, and magnetic resonance imaging (MRI). However, these methods have a common drawback that is inappropriate for measuring NVU elements at high resolution. Some technological advances with high spatial and temporal resolution have recently been applied to measure neuronal and vascular dynamics in the brain, 146 but they have scarcely been used for investigating the spinal cord. Next, we review the methods that can be applied to monitoring NVU changes:
(1) Intrinsic optical signal (IOS) imaging. The first intraoperative IOS imaging of the human brain was reported by Haglund’s group to monitor stimulation-evoked epileptiform discharges and cognitively evoked functional activity in the cerebral cortex. 147 IOS detects changes in light reflectance and the absorption rate of oxyhemoglobin and deoxyhemoglobin to provide information about SCBF, oxygenation, and metabolism. In particular, IOS is commonly used in vivo to provide hemodynamic information in response to simultaneous neuronal activity changes through neurovascular coupling. After electrically stimulating the peripheral nerves, changes in optical reflectance can be recorded simultaneously in the dorsal spinal cord. 148 The responses in the spinal cord increase gradually as the current intensity of the peripheral stimulus increases. Whereas the stimulus intensity has a maximal level, the relationship between stimulus and response is nonlinear out of this range. 149
(2) BOLD-fMRI. The most common form of functional MRI relies on detecting changes in different magnetic properties of oxyhemoglobin and deoxyhemoglobin, called the blood oxygenation level-dependent (BOLD) signal. BOLD-fMRI provides indirect measurements of functional activation of neuronal networks through neurovascular coupling via direct measurement of transient changes in tissue perfusion, blood-volume changes, or oxygen concentration. 150 MRI is the most preferred noninvasive method applied clinically for the spinal cord, as gray and white matter and adjacent structures in the spinal cord can be visualized. Task-related or peripheral stimuli-related functional responses in the spinal cord can be observed under BOLD-fMRI in humans, which can be used to predict motor and sensory recovery after SCI. 123 However, spinal BOLD-fMRI has low spatial specificity because of physiological motion, such as breathing and heartbeat, and predominantly draining veins on the surface of the spinal cord. 151
(3) Optical coherence tomography (OCT). An intrathecal OCT catheter can be used to observe the parenchyma, subarachnoid space, epidural vessels, dentate ligaments, and rootlets with an excellent minimally invasive visualization. Based on the interference pattern of low-coherence broadband light backscattered off the neural tissue, OCT can monitor the moving red blood cells as intrinsic contrast agents to directly measure SCBF. Furthermore, Doppler or speckle variance OCT imaging enables real-time visualization of SCBF in the posterior spinal venous vessels. OCT employing diffuse optical and correlation spectroscopies can also record the SCBF and oxygenation of the spinal cord in real time under various conditions. 152
(4) Laser speckle contrast imaging (LSCI). LSCI detects the speckle contrast signals induced by the movement of red blood cells. This technique can monitor SCBF changes with an excellent spatial and temporal resolution by an ultrafast detector in a noncontact CCD camera. This wide-angle scanning technique can monitor spinal cord blood perfusion after SCI in animal models and patients in a full vision field intraoperatively. 153
(5) Photoacoustic microscopy (PAM). PAM is a hybrid imaging technique based on the photoacoustic effect, combining the advantages of optical and ultrasound techniques and providing high ultrasonic spatial resolution. 146 A photoacoustic signal is generated after inducing pulsed laser energy, causing transient thermoelastic expansion of biological tissue that consequently emits high-frequency acoustic waves detectable by an ultrasound transducer. Imaging of blood optical absorption by PAM at multiple appropriately selected wavelengths can probe changes in hemoglobin concentration, blood volume, and hemoglobin oxygen saturation, along with functional hemodynamic responses to peripheral stimulation. 154 This method can also be utilized to assess tissue loss, guide microinjection, or trace exogenous contrast agents in the spinal cord.
(6) Functional ultrasound imaging (fUSI). fUSI is a recently developed Doppler ultrasound method using a single plane-wave emission as one focused beam to obtain centimeter-level images and coherently sum the set of images from tilted planar illuminations. This ‘compound' ultrasonic image has better resolution and lower noise than other functional imaging. This device can image the microvascular topology and cerebral hemodynamics in vivo with 100 μm resolution, tens of frames per second, and a penetration depth of approximately 8–9 mm in the rodent brain. 155 The fUSI can record the hemodynamic response to neuronal activation induced by peripheral natural or electrical stimulations at a restricted area of approximately 1 mm in the contralateral dorsal horn of the spinal cord. 156
(7) Two-photon fluorescent laser scanning microscopy (2PLSM). 2PLSM is based on the nonlinear excitation of a single fluorescent molecule by two nearly coincident photons, having a great advantage over single-photon fluorescence microscopy. Because of the longer wavelength and lower energy of exciting light, 2PLSM has less laser-induced damage to the tissue and less photobleaching of the imaged fluorophores. In addition, longwave length and low-frequency light is less scattered in the tissue, which enables deeper tissue penetration. One major limitation of 2PLSM is that the observed object needs fluorescence labeling. By probes or transgenic labeling, the electrical activity of neurons can be monitored directly with voltage-sensitive indicators or indirectly with calcium-sensitive and neurotransmitter-sensitive indicators. 146 Methodologically, measurement of hemodynamic change can be classified into two main categories: changes in perfusion or oxygenation. 2PLSM can fulfill the measurement of blood flow velocity and vascular parameters by intravenously injected fluorescence dye (such as Q-dot or fluorescence-labeled dextran) or the measurement of oxygen saturation by a phosphorescent probe (such as PtP-C343). 157 2PLSM can acquire information from fluorescently labeled elements of the NVU in the spinal cord with a very high spatial and temporal resolution, such as the velocity of a single red blood cell. A two-photon-excitable oxygen sensor probe, such as PtP-C343, can be injected intravenously and imaged under 2PLSM to enable quantitative measurement of oxygen metabolism changes in neurovascular coupling. 158
All these methods have advantages and disadvantages and need to be chosen accordingly. Although having an unsurpassed spatial resolution, the 2PLSM can only image the dorsal spinal cord for limited penetration depth and relatively small imaging volume. PAM or fUSI provides deeper tissue penetration to reach the ventral side, so they are suitable for imaging cord-wide changes in NVU activity. MRI cannot provide sufficient spatial resolution to study individual vascular events. BOLD-fMRI is suitable for monitoring organic variation in blood perfusion but is limited by the long delays between sequential images. 157 2PLSM can directly measure the changes in blood velocity or microvascular diameter and is therefore better for surveying neurovascular coupling. However, except for the LSCI and BOLD-fMRI, which give specific values that can be used to reference the changes in blood perfusion, the results detected by other methods require the assistance of formulas (Poiseuille’s law) to estimate changes in blood perfusion.
NVU dysfunction in SCI
After SCI, the normal communication patterns within the NVU can be severely altered. The interruption of metabolism and angioarchitecture after injury can disturb the integrity of NVU function, leading to inappropriate blood perfusion changes 7 and BSCB disruption. 17 Amending NVU dysfunction after SCI, such as BSCB disruption and ischemia, is crucial in the secondary injury mechanism. 150
Furthermore, assessment of small vessel disease in the spinal cord demonstrated a relationship between barrier impairment and lower tissue perfusion, suggesting that defects in functional elements in the NVU can affect each other. 159 Evidence shows that amyotrophic lateral sclerosis is a neurovascular disease, characterized by BSCB disruption, microhemorrhage, and microcirculation hypoperfusion, 160 similar to the secondary injury in SCI. However, NVU dysfunction in SCI may be more complicated, but related investigations are scarce.
Blood supply dysregulation in SCI
Disrupted autoregulation has been observed in early SCI reports. 161 However, whether this “autoregulation of blood perfusion” is an organic level autogenous perfusion control or managed by the NVU has not been confirmed. The systemic blood circulation deficiency after injury could overwhelm the autoregulation of the spinal cord, covering up the changes in this regulatory mechanism. Generally, despite traumatic blood loss, there is a transient state of spinal shock characterized by paralysis of the muscle pump and denervation of the cardiac pump to reduce effective blood circulation. 15 Severe systemic hypotension occurs with evident autonomic dysfunction and attenuated cardiopulmonary output, especially after cervical injuries, termed autonomic dysreflexia. 110 It is characterized by loss of supraspinal sympathetic control on the heart and decreased cardiac pump function. Systemic vasodilation secondary to loss of sympathetic tone further causes low effective circulating blood volume. 162 Profound hypotension leads to MAP failing to maintain autoregulation, continuously aggravating NVU dysfunction. SCI patients sometimes suffer cognitive impairments probably induced by NVU dysfunction caused by cerebral hypoperfusion, which could be reversed by increasing blood pressure. 163
In addition to these systemic effects, the impaired autoregulatory capacity in spinal vasculature can further render the spinal cord vulnerable to systemic hypotension and contribute to ongoing ischemia. 15 Posttraumatic spinal cord ischemia worsens progressively after several hours and persists for weeks in SCI. 153 Hence, medical evidence-based guidelines suggest a level III recommendation to maintain the MAP between 85 and 90 mmHg for the first seven days following SCI. 164 Regardless of spontaneous neurologic recovery after spinal shock, management of MAP alone cannot provide a stable curative effect on the neurological prognosis of SCI patients, 165 making this issue more inexplicable.
Diverse mechanisms can affect blood flow in the spinal cord, including intraspinal cord pressure, which can even change the blood flow direction in venous vessels. 166 Given that the spinal cord is enveloped in the nonelastic dura and bony canal, it may obey the Monro-Kellie doctrine as in the brain. 167 Namely, the total volume of neural tissues, cerebrospinal fluid, and intraspinal blood are fixed. Increased intraspinal cord pressure after SCI caused by tissue swelling or bleeding may exhaust this compensatory ability. The higher the pathological intraspinal cord pressure is, the more deranged the autoregulation of the spinal cord. The intraspinal cord pressure increases significantly after SCI, 168 and the blood flow perfusion and oxygenation metabolism plummets after SCI and remains chronically diminished. 169
Theoretically, when autoregulation is disturbed in SCI, the arterioles are maximally dilated due to local acidosis and hypoxia, and the perfusion of the spinal cord is directly proportional to the systemic blood pressure. 144 However, autoregulation in the injured spinal segment can be preserved for 1–2 hours after SCI.113,161 During this period, a pathological hemodynamic change characterized as a significant blood flow acceleration in venous vessels occurs quickly after SCI and is maintained for approximately 1–2 hours. The pharmacologically elevating MAP only increases blood perfusion for one more hour before ischemia occurs, while for 4 hours, it is accompanied by cerebrospinal fluid drainage. 170 The time course of autoregulation dysfunction in SCI still needs to be elucidated.
Due to the distribution of radicular arteries, blood flow can ascend or descend within the spinal cord, creating numerous watershed areas of blood supply where opposing vascular currents meet. Watershed areas are more susceptible to ischemia. 166 Perfusion in the injured spinal cord is usually a patch-like pattern intersected by regions of low, intermediate, and high blood flow. 153 However, many reports find that hyperperfusion management by vasoconstriction agents after SCI has no benefit. 145 Some parts of the injury site were only perfused in the systolic period 153 and reversely exacerbated hemorrhage and tissue edema. 167 Despite the unfavorable systemic hypotension, vasodilatation agents can dilate the neighboring nondilated arterioles in the well-perfused regions and steal blood from the injured spinal cord (steal-phenomenon). 153 Interestingly, when a calcium channel blocker (nimodipine), which inhibits the contraction of vascular SMCs, is additionally administered for hyperperfusion management after SCI, local perfusion and electrophysiological output both improve. 171 These studies showed that autoregulation in SCI is far from understood, and the potential pharmacological treatment still needs further testing in SCI.
BSCB disruption in SCI
The BSCB disruption permits proinflammatory substances to enter the spinal parenchyma to amplify the pathophysiological cascades and magnify additional damage. 17 In the murine model, BSCB disruption in the acute SCI usually has biphasic peaks: immediate leakage at the epicenter and adjacent site within several hours and a delayed permeability increment with longer duration. 141 After mechanical impact, BSCB leakage generally starts in 5–15 min at the epicenter17,47 and gradually appears in segments away from the epicenter, even along the whole spinal cord in some cases. 130 The murine models show that the segment located 1 cm away from the epicenter suffered BSCB leakage in 30 minutes and 2 cm away in approximately 3 hours. 172 The caudal segments seem more vulnerable to BSCB disruption than the rostral segments. 140 These phenomena suggest that the mechanism of BSCB disruption may be more complex in SCI. Moreover, the barrier disruption is difficult to quantitate except for measuring the leakage of hematogenous tracers where bleeding could also occur, 47 combining hemorrhage and BSCB disruption together to confuse matters. Some data indicate that BSCB disruption in the early period of SCI is a secondary change after mechanical damage. Pathological hemodynamic changes and leukocyte transmigration disrupt the BSCB after SCI and inhibiting these processes can also attenuate BSCB disruption. Much work is needed to clarify the mechanism of BSCB disruption in SCI according to the current view.
Some early studies suggested that BSCB leakage after SCI is caused by vesicular transport. 172 Electron microscopy showed that large numbers of vesicles appeared in the swollen ECs in capillaries and venules within 6 to 10 min after SCI, but without widening of the paracellular junctions. 173 Several forms of vesicle-based transcytosis exist and allow particular macromolecules to cross the endothelium, which can be classified into two categories: clathrin-mediated and caveolae-mediated. 47 The former is receptor-mediated to transfer insulin and transferrin; the latter is adsorptive transcytosis, where charged interactions between the molecule and plasma membrane facilitate its entry. 174 However, vesicular transport abnormalities seem not to be primarily responsible for BSCB disruption after SCI. Because transcytosis as an active transport process is low-rate, selective, and energy dependent, 48 it seems not to correlate with the extensive BSCB leakage of nonselective tracers of varied size in SCI. 17 Nevertheless, there are no further data to prove whether these vesicles are influx or efflux because vesicles are bidirectionally transported or whether these vesicles are caveolae, 175 which is closely related to neurovascular coupling. 176 A recent report found many junctional discontinuities emerging on TJs 15 minutes after SCI, and reducing these junctional discontinuities can decrease BSCB leakage. Combined with the previous electron microscopy data demonstrating no widening of the paracellular junctions, 173 these results probably indicate that not all paracellular junctions are disintegrated in the BSCB leakage after SCI.
Many groups have suggested the degeneration of TJs as the reason for BSCB disruption in SCI, using the expression level of TJs as the indicator for BSCB function. 177 However, most results report that TJs are downregulated at approximately 12–24 h after SCI. 178 The expression of TJs remains unchanged in the early period of SCI when BSCB disruption occurs. Sometimes, a decrease in TJs expression may reflect that BSCB damage is more severe than paracellular leakage, such as whole endothelial cell loss. 130 Therefore, the related treatment to reverse the downregulation of TJs requires more caution when applied to clinical trials, especially in the acute period of SCI.
Conclusion and future directions
Today’s view of the NVU has been broadly amplified. The BCNSB is constantly regarded as a functional configuration in the NVU. Despite the significant similarity of the NVU between the brain and spinal cord, investigation of the NVU in the spinal cord lags far behind that in the brain. Minor differences can be observed in the major elements of the NVU from the limited data available. The vascular anatomy of the spinal cord is markedly different from that of the brain, and the blood flow in the spinal cord is more variable and fluctuating, indicating a more sophisticated autoregulation mechanism in the spinal cord. NMDA receptor-mediated neurovascular coupling is less predominant in the spinal cord. Moreover, the permeability of the BSCB is physiologically higher than that of the BBB, probably because the coverage of pericytes and the expression of some paracellular junctional proteins are inherently lower than those of the BBB. More studies are needed to explore the spinal cord NVU.
Further, in SCI, impaired NVU function could be involved in secondary injury and exacerbate severity. Models of SCI should be weighed to explore the underlying mechanisms contributing to NVU dysfunction. The development of therapies to prevent and treat NVU dysfunction in SCI requires targeted exploration of the injury mechanisms of the NVU.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (81971160) and the Peking University Medicine Seed Fund for Interdisciplinary Research (BMU2018MX021).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Fang Zhou https://orcid.org/0000-0002-7775-069X
References
- 1.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 2011; 12: 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sweeney MD, Zhao Z, Montagne A, et al. Blood-brain barrier: from physiology to disease and back. Physiol Rev 2019; 99: 21–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan L, Chow BW, Gu C. Neuronal regulation of the blood-brain barrier and neurovascular coupling. Nat Rev Neurosci 2020; 21: 416–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinhold AK, Rittner HL. Barrier function in the peripheral and central nervous system – a review. Pflugers Arch 2017; 469: 123–134. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Z, Nelson AR, Betsholtz C, et al. Establishment and dysfunction of the blood-brain barrier. Cell 2015; 163: 1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008; 57: 178–201. [DOI] [PubMed] [Google Scholar]
- 7.Sandsmark DK, Bashir A, Wellington CL, et al. Cerebral microvascular injury: a potentially treatable endophenotype of traumatic brain injury-induced neurodegeneration. Neuron 2019; 103: 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liebner S, Dijkhuizen RM, Reiss Y, et al. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol 2018; 135: 311–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran AP, Warren PM, Silver J. The biology of regeneration failure and success after spinal cord injury. Physiol Rev 2018; 98: 881–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 2017; 96: 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Candelario-Jalil E, Dijkhuizen RM, Magnus T. Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke 2022; 53: 1473–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Xiong X, Zhang L, et al. Neurovascular unit: a critical role in ischemic stroke. CNS Neurosci Ther 2021; 27: 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Wang Y, Yan XL, et al. Pathological changes in neurovascular units: lessons from cases of vascular dementia. CNS Neurosci Ther 2021; 27: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie L, Lu B, Ma Y, et al. The 100 most-cited articles about the role of neurovascular unit in stroke 2001-2020: a bibliometric analysis. CNS Neurosci Ther 2021; 27: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers 2017; 3: 17018. [DOI] [PubMed] [Google Scholar]
- 16.Kumar H, Ropper AE, Lee SH, et al. Propitious therapeutic modulators to prevent blood-spinal cord barrier disruption in spinal cord injury. Mol Neurobiol 2017; 54: 3578–3590. [DOI] [PubMed] [Google Scholar]
- 17.Bartanusz V, Jezova D, Alajajian B, et al. The blood-spinal cord barrier: morphology and clinical implications. Ann Neurol 2011; 70: 194–206. [DOI] [PubMed] [Google Scholar]
- 18.Claassen J, Thijssen DHJ, Panerai RB, et al. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev 2021; 101: 1487–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Claassen JA, Meel-van den Abeelen AS, Simpson DM, et al. Transfer function analysis of dynamic cerebral autoregulation: a white paper from the international cerebral autoregulation research network. J Cereb Blood Flow Metab 2016; 36: 665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips AA, Chan FH, Zheng MM, et al. Neurovascular coupling in humans: physiology, methodological advances and clinical implications. J Cereb Blood Flow Metab 2016; 36: 647–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willie CK, Tzeng YC, Fisher JA, et al. Integrative regulation of human brain blood flow. J Physiol 2014; 592: 841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatterjee S, Fujiwara K, Perez NG, et al. Mechanosignaling in the vasculature: emerging concepts in sensing, transduction and physiological responses. Am J Physiol Heart Circ Physiol 2015; 308: H1451–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaeffer S, Iadecola C. Revisiting the neurovascular unit. Nat Neurosci 2021; 24: 1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mastorakos P, McGavern D. The anatomy and immunology of vasculature in the central nervous system. Sci Immunol 2019; 4: eaav0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otsu Y, Couchman K, Lyons DG, et al. Calcium dynamics in astrocyte processes during neurovascular coupling. Nat Neurosci 2015; 18: 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra A, Reynolds JP, Chen Y, et al. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci 2016; 19: 1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kisler K, Nelson AR, Montagne A, et al. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci 2017; 18: 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathiisen TM, Lehre KP, Danbolt NC, et al. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 2010; 58: 1094–1103. [DOI] [PubMed] [Google Scholar]
- 29.Langen UH, Ayloo S, Gu C. Development and cell biology of the Blood-Brain barrier. Annu Rev Cell Dev Biol 2019; 35: 591–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci 2009; 32: 160–169. [DOI] [PubMed] [Google Scholar]
- 31.Simard M, Arcuino G, Takano T, et al. Signaling at the gliovascular interface. J Neurosci 2003; 23: 9254–9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacVicar BA, Newman EA. Astrocyte regulation of blood flow in the brain. Cold Spring Harb Perspect Biol 2015; 7: a020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zonta M, Angulo MC, Gobbo S, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 2003; 6: 43–50. [DOI] [PubMed] [Google Scholar]
- 34.Grutzendler J, Nedergaard M. Cellular control of brain capillary blood flow: in vivo imaging veritas. Trends Neurosci 2019; 42: 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu X, Chen W, Volkow ND, et al. Synchronized astrocytic Ca(2+) responses in neurovascular coupling during somatosensory stimulation and for the resting state. Cell Rep 2018; 23: 3878–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jukkola P, Guerrero T, Gray V, et al. Astrocytes differentially respond to inflammatory autoimmune insults and imbalances of neural activity. Acta Neuropathol Commun 2013; 1: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filosa JA, Bonev AD, Straub SV, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci 2006; 9: 1397–1403. [DOI] [PubMed] [Google Scholar]
- 38.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 2004; 431: 195–199. [DOI] [PubMed] [Google Scholar]
- 39.Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron 2011; 71: 782–797. [DOI] [PubMed] [Google Scholar]
- 40.Attwell D, Buchan AM, Charpak S, et al. Glial and neuronal control of brain blood flow. Nature 2010; 468: 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chow BW, Gu C. Gradual suppression of transcytosis governs functional blood-retinal barrier formation. Neuron 2017; 93: 1325–1333.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanmarco LM, Polonio CM, Wheeler MA, et al. Functional immune cell-astrocyte interactions. J Exp Med 2021; 218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alvarez JI, Dodelet-Devillers A, Kebir H, et al. The hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 2011; 334: 1727–1731. [DOI] [PubMed] [Google Scholar]
- 44.Argaw AT, Asp L, Zhang J, et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest 2012; 122: 2454–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol 2015; 7: a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otani T, Furuse M. Tight junction structure and function revisited. Trends Cell Biol 2020; 30: 805–817. [DOI] [PubMed] [Google Scholar]
- 47.Wettschureck N, Strilic B, Offermanns S. Passing the vascular barrier: Endothelial signaling processes controlling extravasation. Physiol Rev 2019; 99: 1467–1525. [DOI] [PubMed] [Google Scholar]
- 48.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature 2003; 422: 37–44. [DOI] [PubMed] [Google Scholar]
- 49.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem 2009; 78: 857–902. [DOI] [PubMed] [Google Scholar]
- 50.van der Pol E, Boing AN, Harrison P, et al. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 2012; 64: 676–705. DOI: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 51.Zeiler FA, Thelin EP, Donnelly J, et al. Genetic drivers of cerebral blood flow dysfunction in TBI: a speculative synthesis. Nat Rev Neurol 2019; 15: 25–39. [DOI] [PubMed] [Google Scholar]
- 52.Kis B, Chen L, Ueta Y, et al. Autocrine peptide mediators of cerebral endothelial cells and their role in the regulation of blood-brain barrier. Peptides 2006; 27: 211–222. [DOI] [PubMed] [Google Scholar]
- 53.Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol 2014; 34: 2191–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacob M, Chappell D, Becker BF. Regulation of blood flow and volume exchange across the microcirculation. Crit Care 2016; 20: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tarbell JM, Simon SI, Curry FR. Mechanosensing at the vascular interface. Annu Rev Biomed Eng 2014; 16: 505–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tzima E, Irani-Tehrani M, Kiosses WB, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005; 437: 426–431. [DOI] [PubMed] [Google Scholar]
- 57.Baratchi S, Khoshmanesh K, Woodman OL, et al. Molecular sensors of blood flow in endothelial cells. Trends Mol Med 2017; 23: 850–868. [DOI] [PubMed] [Google Scholar]
- 58.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol 1998; 508: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harraz OF, Hill-Eubanks D, Nelson MT. PIP2: a critical regulator of vascular ion channels hiding in plain sight. Proc Natl Acad Sci U S A 2020; 117: 20378–20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hill-Eubanks DC, Gonzales AL, Sonkusare SK, et al. Vascular TRP channels: performing under pressure and going with the flow. Physiology (Bethesda) 2014; 29: 343–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pascual O, Casper KB, Kubera C, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science 2005; 310: 113–116. [DOI] [PubMed] [Google Scholar]
- 62.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci 2011; 14: 1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daneman R, Zhou L, Kebede AA, et al. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010; 468: 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cardoso FL, Brites D, Brito MA. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev 2010; 64: 328–363. [DOI] [PubMed] [Google Scholar]
- 66.Berthiaume AA, Schmid F, Stamenkovic S, et al. Pericyte remodeling is deficient in the aged brain and contributes to impaired capillary flow and structure. Nat Commun 2022; 13: 5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature 2010; 468: 557–561. [DOI] [PubMed] [Google Scholar]
- 68.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 2016; 19: 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hori S, Ohtsuki S, Hosoya K, et al. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through tie-2 activation in vitro. J Neurochem 2004; 89: 503–513. [DOI] [PubMed] [Google Scholar]
- 70.Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010; 68: 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Attwell D, Mishra A, Hall CN, et al. What is a pericyte? J Cereb Blood Flow Metab 2016; 36: 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kisler K, Nelson AR, Rege SV, et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat Neurosci 2017; 20: 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rungta RL, Chaigneau E, Osmanski BF, et al. Vascular compartmentalization of functional hyperemia from the synapse to the pia. Neuron 2018; 99: 362–375 e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gordon GR, Choi HB, Rungta RL, et al. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 2008; 456: 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Winkler EA, Sengillo JD, Bell RD, et al. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cereb Blood Flow Metab 2012; 32: 1841–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y, Lucas-Osma AM, Black S, et al. Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat Med 2017; 23: 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carvey PM, Hendey B, Monahan AJ. The blood-brain barrier in neurodegenerative disease: a rhetorical perspective. J Neurochem 2009; 111: 291–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Poschl E, Schlotzer-Schrehardt U, Brachvogel B, et al. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 2004; 131: 1619–1628. [DOI] [PubMed] [Google Scholar]
- 79.Nirwane A, Yao Y. Cell-specific expression and function of laminin at the neurovascular unit. J Cereb Blood Flow Metab 2022; 42: 1979–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gautam J, Cao Y, Yao Y. Pericytic laminin maintains blood-brain barrier integrity in an age-dependent manner. Transl Stroke Res 2020; 11: 228–242. [DOI] [PubMed] [Google Scholar]
- 81.Menezes MJ, McClenahan FK, Leiton CV, et al. The extracellular matrix protein laminin alpha2 regulates the maturation and function of the blood-brain barrier. J Neurosci 2014; 34: 15260–15280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamamoto H, Ehling M, Kato K, et al. Integrin beta1 controls VE-cadherin localization and blood vessel stability. Nat Commun 2015; 6: 6429. [DOI] [PubMed] [Google Scholar]
- 83.Milich LM, Ryan CB, Lee JK. The origin, fate, and contribution of macrophages to spinal cord injury pathology. Acta Neuropathol 2019; 137: 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Almeida LGN, Thode H, Eslambolchi Y, et al. Matrix metalloproteinases: from molecular mechanisms to physiology, pathophysiology, and pharmacology. Pharmacol Rev 2022; 74: 712–768. [DOI] [PubMed] [Google Scholar]
- 85.Rempe RG, Hartz AMS, Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J Cereb Blood Flow Metab 2016; 36: 1481–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Loffek S, Schilling O, Franzke CW. Series “matrix metalloproteinases in lung health and disease”: biological role of matrix metalloproteinases: a critical balance. Eur Respir J 2011; 38: 191–208. [DOI] [PubMed] [Google Scholar]
- 87.Abdul-Muneer PM, Pfister BJ, Haorah J, et al. Role of matrix metalloproteinases in the pathogenesis of traumatic brain injury. Mol Neurobiol 2016; 53: 6106–6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol 2009; 8: 205–216. [DOI] [PubMed] [Google Scholar]
- 89.Agrawal S, Anderson P, Durbeej M, et al. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med 2006; 203: 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Figueiredo CA, Steffen J, Morton L, et al. Immune response and pathogen invasion at the choroid plexus in the onset of cerebral toxoplasmosis. J Neuroinflammation 2022; 19: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang H, Chang M, Hansen CN, et al. Role of matrix metalloproteinases and therapeutic benefits of their inhibition in spinal cord injury. Neurotherapeutics 2011; 8: 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Veeravalli KK, Dasari VR, Rao JS. Regulation of proteases after spinal cord injury. J Neurotrauma 2012; 29: 2251–2262. [DOI] [PubMed] [Google Scholar]
- 93.Verslegers M, Lemmens K, Van Hove I, et al. Matrix metalloproteinase-2 and -9 as promising benefactors in development, plasticity and repair of the nervous system. Prog Neurobiol 2013; 105: 60–78. [DOI] [PubMed] [Google Scholar]
- 94.Trivedi A, Zhang H, Ekeledo A, et al. Deficiency in matrix metalloproteinase-2 results in long-term vascular instability and regression in the injured mouse spinal cord. Exp Neurol 2016; 284: 50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shindo A, Liang AC, Maki T, et al. Subcortical ischemic vascular disease: roles of oligodendrocyte function in experimental models of subcortical white-matter injury. J Cereb Blood Flow Metab 2016; 36: 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Niu J, Tsai HH, Hoi KK, et al. Aberrant oligodendroglial-vascular interactions disrupt the blood-brain barrier, triggering CNS inflammation. Nat Neurosci 2019; 22: 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haruwaka K, Ikegami A, Tachibana Y, et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun 2019; 10: 5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bisht K, Okojie KA, Sharma K, et al. Capillary-associated microglia regulate vascular structure and function through PANX1-P2RY12 coupling in mice. Nat Commun 2021; 12: 5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dufort C, Wang Y, Hu X. Microglia: Active participants in brain capillary function. J Cereb Blood Flow Metab 2022; 42: 2161–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev 1959; 39: 183–238. [DOI] [PubMed] [Google Scholar]
- 101.Diehl RR, Linden D, Lucke D, et al. Phase relationship between cerebral blood flow velocity and blood pressure. a clinical test of autoregulation. Stroke 1995; 26: 1801–1804. [DOI] [PubMed] [Google Scholar]
- 102.Hickey R, Albin MS, Bunegin L, et al. Autoregulation of spinal cord blood flow: is the cord a microcosm of the brain? Stroke 1986; 17: 1183–1189. [DOI] [PubMed] [Google Scholar]
- 103.Halstead JC, Wurm M, Etz C, et al. Preservation of spinal cord function after extensive segmental artery sacrifice: regional variations in perfusion. Ann Thorac Surg 2007; 84: 789–794. [DOI] [PubMed] [Google Scholar]
- 104.Kobrine AI, Doyle TF, Newby N, et al. Preserved autoregulation in the rhesus spinal cord after high cervical cord section. J Neurosurg 1976; 44: 425–428. [DOI] [PubMed] [Google Scholar]
- 105.Piche M, Paquette T, Leblond H. Tight neurovascular coupling in the spinal cord during nociceptive stimulation in intact and spinal rats. Neuroscience 2017; 355: 1–8. [DOI] [PubMed] [Google Scholar]
- 106.van Beek AH, Claassen JA, Rikkert MG, et al. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab 2008; 28: 1071–1085. [DOI] [PubMed] [Google Scholar]
- 107.Greenfield JC, Jr., Rembert JC, Tindall GT. Transient changes in cerebral vascular resistance during the Valsalva maneuver in man. Stroke 1984; 15: 76–79. [DOI] [PubMed] [Google Scholar]
- 108.Tiecks FP, Lam AM, Aaslid R, et al. Comparison of static and dynamic cerebral autoregulation measurements. Stroke 1995; 26: 1014–1019. [DOI] [PubMed] [Google Scholar]
- 109.de Jong DLK, Tarumi T, Liu J, et al. Lack of linear correlation between dynamic and steady-state cerebral autoregulation. J Physiol 2017; 595: 5623–5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Phillips AA, Ainslie PN, Krassioukov AV, et al. Regulation of cerebral blood flow after spinal cord injury. J Neurotrauma 2013; 30: 1551–1563. [DOI] [PubMed] [Google Scholar]
- 111.van Tuijl RJ, Ruigrok YM, Velthuis BK, et al. Velocity pulsatility and arterial distensibility along the internal carotid artery. J Am Heart Assoc 2020; 9: e016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Belz GG. Elastic properties and windkessel function of the human aorta. Cardiovasc Drugs Ther 1995; 9: 73–83. [DOI] [PubMed] [Google Scholar]
- 113.Martirosyan NL, Feuerstein JS, Theodore N, et al. Blood supply and vascular reactivity of the spinal cord under normal and pathological conditions. J Neurosurg Spine 2011; 15: 238–251. [DOI] [PubMed] [Google Scholar]
- 114.Pournaras CJ, Rungger-Brandle E, Riva CE, et al. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res 2008; 27: 284–330. [DOI] [PubMed] [Google Scholar]
- 115.Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab 2012; 32: 1107–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Frederiksen SD, Haanes KA, Warfvinge K, et al. Perivascular neurotransmitters: Regulation of cerebral blood flow and role in primary headaches. J Cereb Blood Flow Metab 2019; 39: 610–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Young W. Sympathectomy and spinal cord blood flow. J Neurosurg 1992; 77: 654–655. [PubMed] [Google Scholar]
- 118.Hartmann DA, Coelho-Santos V, Shih AY. Pericyte control of blood flow across microvascular zones in the central nervous system. Annu Rev Physiol 2022; 84: 331–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Montalbano MJ, Loukas M, Oskouian RJ, et al. Innervation of the blood vessels of the spinal cord: a comprehensive review. Neurosurg Rev 2018; 41: 733–735. [DOI] [PubMed] [Google Scholar]
- 120.Tang S, Cuellar CA, Song P, et al. Changes in spinal cord hemodynamics reflect modulation of spinal network with different parameters of epidural stimulation. Neuroimage 2020; 221: 117183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kristensen JD, Karlsten R, Gordh T. Laser-Doppler evaluation of spinal cord blood flow after intrathecal administration of an N-methyl-D-aspartate antagonist in rats. Anesth Analg 1994; 78: 925–931. [DOI] [PubMed] [Google Scholar]
- 122.Acharya D, Ruesch A, Schmitt S, et al. Changes in neurovascular coupling with cerebral perfusion pressure indicate a link to cerebral autoregulation. J Cereb Blood Flow Metab 2022; 42: 1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stroman PW, Khan HS, Bosma RL, et al. Changes in pain processing in the spinal cord and brainstem after spinal cord injury characterized by functional magnetic resonance imaging. J Neurotrauma 2016; 33: 1450–1460. [DOI] [PubMed] [Google Scholar]
- 124.Sprenger C, Finsterbusch J, Buchel C. Spinal cord-midbrain functional connectivity is related to perceived pain intensity: a combined spino-cortical FMRI study. J Neurosci 2015; 35: 4248–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sharma V, He JW, Narvenkar S, et al. Quantification of light reflectance spectroscopy and its application: determination of hemodynamics on the rat spinal cord and brain induced by electrical stimulation. Neuroimage 2011; 56: 1316–1328. [DOI] [PubMed] [Google Scholar]
- 126.Davson H. Review lecture. The blood-brain barrier. J Physiol 1976; 255: 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Andersson PB, Perry VH, Gordon S. Intracerebral injection of proinflammatory cytokines or leukocyte chemotaxins induces minimal myelomonocytic cell recruitment to the parenchyma of the Central nervous system. J Exp Med 1992; 176: 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol 2017; 18: 123–131. [DOI] [PubMed] [Google Scholar]
- 129.Abbott NJ, Pizzo ME, Preston JE, et al. The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic' system? Acta Neuropathol 2018; 135: 387–407. [DOI] [PubMed] [Google Scholar]
- 130.Erickson MA, Banks WA. Neuroimmune axes of the blood-brain barriers and blood-brain interfaces: bases for physiological regulation, disease states, and pharmacological interventions. Pharmacol Rev 2018; 70: 278–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Banks WA. The blood-brain barrier as an endocrine tissue. Nat Rev Endocrinol 2019; 15: 444–455. [DOI] [PubMed] [Google Scholar]
- 132.Ghersi-Egea JF, Strazielle N, Catala M, et al. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol 2018; 135: 337–361. [DOI] [PubMed] [Google Scholar]
- 133.Kastin AJ, Akerstrom V. Glucose and insulin increase the transport of leptin through the blood-brain barrier in normal mice but not in streptozotocin-diabetic mice. Neuroendocrinology 2001; 73: 237–242. [DOI] [PubMed] [Google Scholar]
- 134.Zihni C, Mills C, Matter K, et al. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol 2016; 17: 564–580. [DOI] [PubMed] [Google Scholar]
- 135.Hajal C, Le Roi B, Kamm RD, et al. Biology and models of the blood-brain barrier. Annu Rev Biomed Eng 2021; 23: 359–384. [DOI] [PubMed] [Google Scholar]
- 136.Kutuzov N, Flyvbjerg H, Lauritzen M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood-brain barrier. Proc Natl Acad Sci U S A 2018; 115: E9429–E9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mautes AE, Weinzierl MR, Donovan F, et al. Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys Ther 2000; 80: 673–687. [PubMed] [Google Scholar]
- 138.Saunders NR, Dreifuss JJ, Dziegielewska KM, et al. The rights and wrongs of blood-brain barrier permeability studies: a walk through 100 years of history. Front Neurosci 2014; 8: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang B, Gensel JC. Is neuroinflammation in the injured spinal cord different than in the brain? Examining intrinsic differences between the brain and spinal cord. Exp Neurol 2014; 258: 112–120. [DOI] [PubMed] [Google Scholar]
- 140.Sharma HS. Pathophysiology of blood-spinal cord barrier in traumatic injury and repair. Curr Pharm Des 2005; 11: 1353–1389. [DOI] [PubMed] [Google Scholar]
- 141.Neuwelt E, Abbott NJ, Abrey L, et al. Strategies to advance translational research into brain barriers. Lancet Neurol 2008; 7: 84–96. [DOI] [PubMed] [Google Scholar]
- 142.Uchida Y, Sumiya T, Tachikawa M, et al. Involvement of claudin-11 in disruption of blood-brain, -spinal cord, and -arachnoid barriers in multiple sclerosis. Mol Neurobiol 2019; 56: 2039–2056. [DOI] [PubMed] [Google Scholar]
- 143.Uchida Y, Yagi Y, Takao M, et al. Comparison of absolute protein abundances of transporters and receptors among blood-brain barriers at different cerebral regions and the blood-spinal cord barrier in humans and rats. Mol Pharm 2020; 17: 2006–2020. [DOI] [PubMed] [Google Scholar]
- 144.Mortazavi MM, Verma K, Harmon OA, et al. The microanatomy of spinal cord injury: a review. Clin Anat 2015; 28: 27–36. [DOI] [PubMed] [Google Scholar]
- 145.Guha A, Tator CH, Rochon J. Spinal cord blood flow and systemic blood pressure after experimental spinal cord injury in rats. Stroke 1989; 20: 372–377. [DOI] [PubMed] [Google Scholar]
- 146.Urban A, Golgher L, Brunner C, et al. Understanding the neurovascular unit at multiple scales: advantages and limitations of multi-photon and functional ultrasound imaging. Adv Drug Deliv Rev 2017; 119: 73–100. [DOI] [PubMed] [Google Scholar]
- 147.Haglund MM, Ojemann GA, Hochman DW. Optical imaging of epileptiform and functional activity in human cerebral cortex. Nature 1992; 358: 668–671. [DOI] [PubMed] [Google Scholar]
- 148.Sasaki S, Sato K, Shinomiya K, et al. Postnatal changes in intrinsic optical responses to peripheral nerve stimulation in the in vivo rat spinal cord. Neuroimage 2003; 20: 2126–2134. [DOI] [PubMed] [Google Scholar]
- 149.Sasaki S, Yazawa I, Miyakawa N, et al. Optical imaging of intrinsic signals induced by peripheral nerve stimulation in the in vivo rat spinal cord. Neuroimage 2002; 17: 1240–1255. [DOI] [PubMed] [Google Scholar]
- 150.David G, Mohammadi S, Martin AR, et al. Traumatic and nontraumatic spinal cord injury: pathological insights from neuroimaging. Nat Rev Neurol 2019; 15: 718–731. [DOI] [PubMed] [Google Scholar]
- 151.Leitch JK, Figley CR, Stroman PW. Applying functional MRI to the spinal cord and brainstem. Magn Reson Imaging 2010; 28: 1225–1233. [DOI] [PubMed] [Google Scholar]
- 152.Mesquita RC, D'Souza A, Bilfinger TV, et al. Optical monitoring and detection of spinal cord ischemia. PLoS One 2013; 8: e83370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gallagher MJ, Hogg FRA, Zoumprouli A, et al. Spinal cord blood flow in patients with acute spinal cord injuries. J Neurotrauma 2019; 36: 919–929. [DOI] [PubMed] [Google Scholar]
- 154.Liao LD, Lin CT, Shih YY, et al. Transcranial imaging of functional cerebral hemodynamic changes in single blood vessels using in vivo photoacoustic microscopy. J Cereb Blood Flow Metab 2012; 32: 938–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Martinez de Paz JM, Mace E. Functional ultrasound imaging: a useful tool for functional connectomics? Neuroimage 2021; 245: 118722. [DOI] [PubMed] [Google Scholar]
- 156.Claron J, Hingot V, Rivals I, et al. Large-scale functional ultrasound imaging of the spinal cord reveals in-depth spatiotemporal responses of spinal nociceptive circuits in both normal and inflammatory states. Pain 2021; 162: 1047–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fumagalli S, Ortolano F, De Simoni MG. A close look at brain dynamics: cells and vessels seen by in vivo two-photon microscopy. Prog Neurobiol 2014; 121: 36–54. [DOI] [PubMed] [Google Scholar]
- 158.Lecoq J, Parpaleix A, Roussakis E, et al. Simultaneous two-photon imaging of oxygen and blood flow in deep cerebral vessels. Nat Med 2011; 17: 893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wong SM, Jansen JFA, Zhang CE, et al. Blood-brain barrier impairment and hypoperfusion are linked in cerebral small vessel disease. Neurology 2019; 92: e1669–e1677. [DOI] [PubMed] [Google Scholar]
- 160.Rule RR, Schuff N, Miller RG, et al. Gray matter perfusion correlates with disease severity in ALS. Neurology 2010; 74: 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Senter HJ, Venes JL. Loss of autoregulation and posttraumatic ischemia following experimental spinal cord trauma. J Neurosurg 1979; 50: 198–206. [DOI] [PubMed] [Google Scholar]
- 162.Partida E, Mironets E, Hou S, et al. Cardiovascular dysfunction following spinal cord injury. Neural Regen Res 2016; 11: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Phillips AA, Warburton DE, Ainslie PN, et al. Regional neurovascular coupling and cognitive performance in those with low blood pressure secondary to high-level spinal cord injury: improved by alpha-1 agonist midodrine hydrochloride. J Cereb Blood Flow Metab 2014; 34: 794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Walters BC, Hadley MN, Hurlbert RJ, et al. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 2013; 60: 82–91. [DOI] [PubMed] [Google Scholar]
- 165.Saadeh YS, Smith BW, Joseph JR, et al. The impact of blood pressure management after spinal cord injury: a systematic review of the literature. Neurosurg Focus 2017; 43: E20. [DOI] [PubMed] [Google Scholar]
- 166.Hernandez-Gerez E, Fleming IN, Parson SH. A role for spinal cord hypoxia in neurodegeneration. Cell Death Dis 2019; 10: 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Saadoun S, Papadopoulos MC. Acute, severe traumatic spinal cord injury: monitoring from the injury site and expansion duraplasty. Neurosurg Clin N Am 2021; 32: 365–376. [DOI] [PubMed] [Google Scholar]
- 168.Khaing ZZ, Cates LN, Fischedick AE, et al. Temporal and spatial evolution of raised intraspinal pressure after traumatic spinal cord injury. J Neurotrauma 2017; 34: 645–651. [DOI] [PubMed] [Google Scholar]
- 169.Meyer BP, Hirschler L, Lee S, et al. Optimized cervical spinal cord perfusion MRI after traumatic injury in the rat. J Cereb Blood Flow Metab 2021; 41: 2010–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Martirosyan NL, Kalani MY, Bichard WD, et al. Cerebrospinal fluid drainage and induced hypertension improve spinal cord perfusion after acute spinal cord injury in pigs. Neurosurgery 2015; 76: 461–468; discussion 468–469. [DOI] [PubMed] [Google Scholar]
- 171.Fehlings MG, Tator CH, Linden RD. The effect of nimodipine and dextran on axonal function and blood flow following experimental spinal cord injury. J Neurosurg 1989; 71: 403–416. [DOI] [PubMed] [Google Scholar]
- 172.Noble LJ, Wrathall JR. Distribution and time course of protein extravasation in the rat spinal cord after contusive injury. Brain Res 1989; 482: 57–66. [DOI] [PubMed] [Google Scholar]
- 173.Griffiths IR, McCulloch M, Crawford RA. Ultrastructural appearances of the spinal microvasculature between 12 hours and 5 days after impact injury. Acta Neuropathol 1978; 43: 205–211. [DOI] [PubMed] [Google Scholar]
- 174.Ayloo S, Gu CH. Transcytosis at the blood-brain barrier. Curr Opin Neurobiol 2019; 57: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Parton RG. Caveolae: Structure, function, and relationship to disease. Annu Rev Cell Dev Biol 2018; 34: 111–136. [DOI] [PubMed] [Google Scholar]
- 176.Chow BW, Nunez V, Kaplan L, et al. Caveolae in CNS arterioles mediate neurovascular coupling. Nature 2020; 579: 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jin LY, Li J, Wang KF, et al. Blood-spinal cord barrier in spinal cord injury: a review. J Neurotrauma 2021; 38: 1203–1224. [DOI] [PubMed] [Google Scholar]
- 178.Chio JCT, Wang J, Badner A, et al. The effects of human immunoglobulin G on enhancing tissue protection and neurobehavioral recovery after traumatic cervical spinal cord injury are mediated through the neurovascular unit. J Neuroinflammation 2019; 16: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]