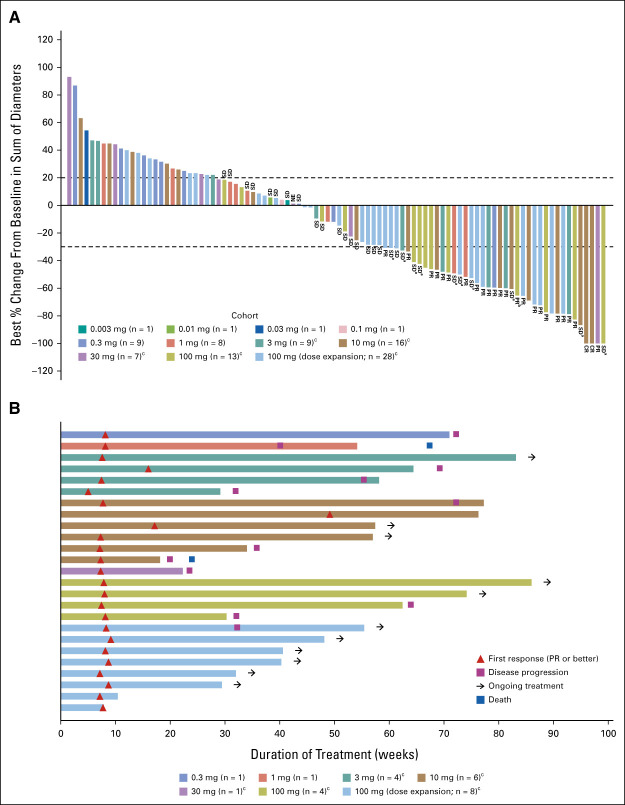
FIG 1.
Response to tarlatamab. (A) Best percent change from baseline in tumor burden (defined by the sum of the longest diameters of all target lesions) in 94 patients whose data cutoff date is at least 9 weeks after the first dose date and for whom postbaseline tumor data were available. aSD, patients had an initial response but did not have confirmation of response on the subsequent scan and bPR, patients had an initial PR and still have potential for future confirmative scans. One confirmed patient in the 100 mg expansion cohort had missing sum of diameters for lesion measurement and was not included in the plot. cStep dosing (ie, 1 mg run-in dose) was used in these cohorts. (B) TTR, the duration of treatment, and patient status as of the data cutoff date according to dose of tarlatamab for all patients with confirmed response (n = 25). CR, complete response; NE, not evaluable; PR, partial response; SD, stable disease; TTR, time to response.