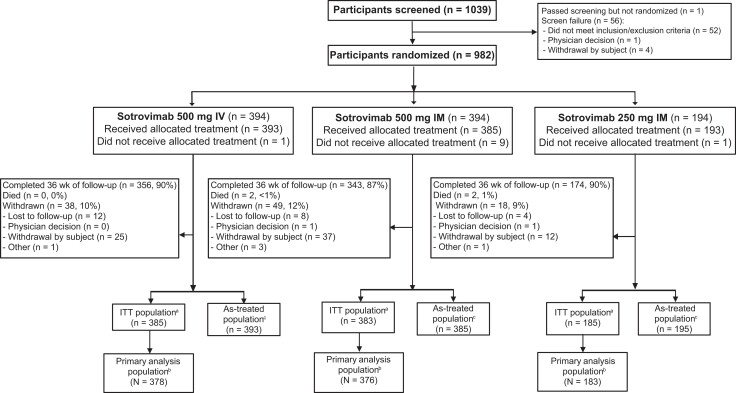
Figure 2.
Patient enrollment and treatment assignment (sotrovimab). aITT population includes all randomly assigned participants, excluding those who were immunocompetent and fully vaccinated under the original protocol. bPrimary analysis population is defined as the ITT population minus those who violated key inclusion/exclusion criteria. cThe as-treated (safety) population includes all participants who received the study treatment. Two participants who were randomized to sotrovimab 500 mg IM received 250 mg IM and are included in the latter as-treated population. IM, intramuscular; IV, intravenous; ITT, intent to treat.