Abstract
Amla (Phyllanthus emblica) has long been used in traditional folk medicine to prevent and cure a variety of inflammatory diseases. In this study, the antioxidant activity (DPPH scavenging and reducing power), anti-inflammatory activity (RBC Membrane Stabilization and 15-LOX inhibition), and anticoagulation activity (Serin protease inhibition and Prothrombin Time assays) of the methanolic extract of amla were conducted. Amla exhibited a substantial amount of phenolic content (TPC: 663.53 mg GAE/g) and flavonoid content (TFC: 418.89 mg GAE/g). A strong DPPH scavenging effect was observed with an IC50 of 311.31 µg/ml as compared to standard ascorbic acid with an IC50 of 130.53 µg/ml. In reducing power assay, the EC50 value of the extract was found to be 196.20 µg/ml compared to standard ascorbic acid (EC50 = 33.83 µg/ml). The IC50 value of the RBC membrane stabilization and 15-LOX assays was observed as 101.08 µg/ml (IC50 of 58.62 µg/ml for standard aspirin) and 195.98 µg/ml (IC50 of 19.62 µg/ml for standard quercetin), respectively. The extract also strongly inhibited serine protease (trypsin) activity with an IC50 of 505.81 µg/ml (IC50 of 295.44 µg/ml for standard quercetin). The blood coagulation time (PTT) was found to be 11.91 min for amla extract and 24.11 min for standard Warfarin. Thus, the findings of an in vitro study revealed that the methanolic extract of amla contains significant antioxidant, anti-inflammatory, and anticoagulation activity. Furthermore, in silico docking and simulation of reported phytochemicals of amla with human 15-LOXA and 15-LOXB were carried out to validate the anti-inflammatory activity of amla. In this analysis, epicatechin and catechin showed greater molecular interaction and were considerably stable throughout the 100 ns simulation with 15-lipoxygenase A (15-LOXA) and 15-lipoxygenase B (15-LOXB) respectively.
Keywords: Antioxidant, Anti-inflammatory, Anticoagulation, Molecular docking, Molecular dynamic simulation
1. Introduction
Reactive oxygen species (ROS) are free radical molecules that are produced as by-products of metabolic processes in the cells, primarily in mitochondria (Thannickal and Fanburg 2000). Ros is necessary for maintaining cell signaling and immune responses at a low concentration, but an elevated generation levels of ROS accumulation can lead to oxidative stress in cellular systems and result in adverse molecular effects (Kabel 2014). Subsequently, these effects are related to various health complications, including diabetes, cancer, inflammation, coronary-respiratory disease, nephrological disease, and neurodegenerative illnesses such as Alzheimer's disease (Birben et al., 2012, Umeno et al., 2017). Increased ROS levels in the cell further induce lipoxygenase (LOX)-mediated lipid peroxidation that utilizes cellular linolic acid or arachidonic acid as substrates and resulting in the production of hydroxide known as hydroperoxy eicosatetraenoic acid (HPETE) (Wisastra and Dekker 2014). Under stress conditions, 15-LOX transforms arachidonic acid into its equivalent lipid peroxides, which are subsequently transformed into a variety of inflammatory mediators (eoxins, 12-Hydroxyeicosatetraenoic Acid, and 15-Hydroxyeicosatetraenoic Acid) in the presence of glutathione. These inflammatory mediators upregulate the production of pro-inflammatory cytokines (Eleftheriadis et al., 2016). Oxidative stress also damages vascular endothelial cells and upregulates tissue factors that activate serine proteases, which are involved in the extrinsic pathway of the coagulation cascade (Lopes-Pires et al., 2021). Exogenous antioxidant consumption has been proposed as a mechanism to overcome oxidative damage and pro-inflammatory conditions (Islam et al., 2020).
Plant-derived extracts and preparations s have been utilized to heal diseases as ethnotherapeutic approaches in Eastern medicine due to their significant therapeutic characteristics for instance- antiviral, antibacterial, antifungal, anti-inflammatory, and anti-cancer properties with only a few adverse effects and toxicity (Rahman et al., 2020, Rahman et al., 2020, Biswas et al., 2021, Dey et al., 2022). Phyllanthus emblica (popularly known as Amla/Amloki/ Amalaki/Indian Gooseberry) is well-known for its coarse nutritional properties, but it also includes a wide range of phytochemicals including tannins, mucic acid and phenolics (Variya et al., 2016, Sohel et al., 2022). Due to its high content of essential amino acids and micronutrients, P. emblica has traditionally been used medicinally and as a nutritional tonic (Srivasuki, 2012, Dey et al., 2022). Preliminary studies have shown that P. emblica contains a variety of pharmacological properties, including antioxidants, anti-inflammatory, hepatoprotective, anticancer, analgesic, antibacterial, antiviral, antidiabetic, laxative, antidiarrheal, neuroprotective, cardioprotective activities (Gaire and Subedi 2014). Several studies reported Chebulagic Acid, Gallic Acid, Chebulinic Acid, Corilagin, Ellagic Acid, Rutin, Kaempferol, Wogonin, Epicatechin, Catechin, Resveratrol, Procyanidin B2, Myricetin, Hamamelitannin, Ethyl Gallate, Methyl Gallate, Quercetin-3-O-Rhamnoside, Ascorbic Acid, Chlorogenic Acid, and Phyllanthin as potential phytochemicals in P. emblica (Yang et al., 2012, Gaire and Subedi, 2014). These phytochemicals are known for their different therapeutic activities.
So far, in general, it is evident that this plant has antioxidants, anticoagulants, and anti-inflammatory activity. Several studies focused on COX-2 inhibitory activity (Islam et al., 2022, Li et al., 2022, Khan et al., 2023). But there is a lack of organized study that focuses on 15-LOX. Few of the above-mentioned phytochemicals are well-known to bind to 15-LOX but the information is sporadic and those studies are not focused only on P. emblica. We hypothesize that these phytochemicals, in combination, might be responsible for the therapeutic properties of P. emblica. So, our study aims to evaluate the antioxidant, anticoagulant, and anti-inflammatory activity of P.emblica fruit extract. We also aim to validate the interaction of potential phytochemicals to human 15-LOXA and 15-LOXB by an in-silico docking study against potential phytochemicals of P.emblica. The molecular interaction will be further assessed for their stability by in-silico molecular dynamic simulation. This research will help us understand the role of certain potential phytochemicals, their interaction with human 15-LOX, in mediating antioxidant, anticoagulant, and anti-inflammatory activity.
2. Materials and method
2.1. Collection of plant material
Fruits of Phyllanthus emblica were purchased from Gollamari local market (https://goo.gl/maps/HdjfgTsg3HfLU1MW9), Khulna, Bangladesh on October 20, 2021.
2.2. Preparation of extract
Before extraction, the amla fruits were cut into pieces and washed thoroughly with distilled water and shade dried for four weeks at ambient temperature. Then fruits of Amla were ground into fine powder and stored in a dry, cool, and dark area in an airtight container. Nearly 20 g of the powdered fruits were extracted using 200 mL of methanol. These mixtures were kept at 25 °C for 72 h with intermittent stirring. Samples were filtered and dried at 55 °C in a rotary evaporator with lower pressure to obtain methanolic crude extract (extract yield 2.53% of the dry plant material).
2.3. Determination of antioxidant activity
2.3.1. DPPH free radical scavenging assay
The DPPH essay was conducted in accordance with (Brand-Williams et al., 1995, Rahman et al., 2021, Hasan et al., 2022). The percentage inhibition of DPPH was determined by using Eqn 1:
| (1) |
2.3.2. Reducing power assay
The reducing power assay was conducted according to the procedure stated by (Afrin et al., 2021). The percentage increase in reducing power was determined by using Eqn 2:
| (2) |
2.4. Determination of phytochemical constitution
2.4.1. Total phenolic content
The total phenolic content assay of the samples was conducted using the Folin–Ciocalteu (FC) reagent, as mentioned by (Rebaya et al., 2015, Zilani et al., 2021). The optical density at 750 nm was taken to determine the concentration of phenolic compounds. A gallic acid standard curve was constructed from the absorbance to quantify the concentration of phenolics in gallic acid equivalents (GAE).
2.4.2. Total flavonoid content
The total flavonoid content (TFC) of the sample was determined using the aluminum chloride spectrophotometric approach described by (Rebaya et al., 2015). The flavonoid content was represented as a quercetin equivalent (QE).
2.4.3. Total tannin content
Total tannin content (TTC) was calculated according to the procedure as stated before by the Polshettiwar et al., 2007 (Polshettiwar et al., 2007).
2.5. Determination of in vitro anti-inflammatory activity
2.5.1. Ethical approval for blood sampling from volunteers for research
5 mL blood samples were collected from each healthy individual (Khulna University students) with the assistance of medical specialists from the Khulna University Medical Center, and all participants provided prior informed consent. The ethical code (KUAEC-2017/08/14) of the Khulna University Research Cell was followed and agreed to for all of the experiments.
2.5.2. Hypotonicity induced human red blood cell membrane stabilization (HRBCMS) assay
To perform this assay, blood samples were taken from healthy volunteers and then transferred to EDTA tubes to prevent clotting. The hypotonicity induced HRBCMS ex-vivo experiment was carried out in accordance with previous study (Yesmin et al., 2020). The percentage of inhibition of hemolysis was determined by using following Eqn 3:
| (3) |
OD1 represents the optical density of an isotonic solution, OD2 the optical density of a hypotonic solution, and OD3 represents the optical density of a hypotonic solution used as the control.
2.5.3. 15-lipoxygenase inhibition assay
The 15-LOX inhibition experiment was performed on the crude extract using lyophilized 15-LOX (Extra pure soybean lipoxigenase bought from Sigma Aldrich, US) in accordance with Wangensteen's original method with minor modifications (Pham et al., 2014).
50–400 g/mL aliquots of extract, standard (Quercetin), or blank (0.1 M PBS) were combined with 975 mL 15-LOX solution (3,000 U/mL; reconstituted with 0.2 M borate buffer; pH 9.0). To initiate the reaction, each tube was filled halfway with 517.5 M linoleic acid substrate solution (reconstituted with 0.2 M borate buffer; pH 9.0). For ten minutes, the solution's absorbance was determined at one-minute intervals.
Absorbance vs time plot for each sample was prepared (ΔAbsorbance/ΔTime) and the slope was measured for enzyme inhibition according to Eqn 4:.
| (4) |
2.6. Determination of in vitro anti-coagulation activity
2.6.1. Serine protease inhibition assay
The serine protease inhibition experiment was conducted according to the procedure stated by (Kunitz, 1947). the percentage of inhibition was determined, using Eqn 2
2.6.2. Prothrombin time (PTT) assay
The PTT was conducted in accordance with the procedure stated by (Biswas et al., 2019) using human fresh blood collected from healthy volunteers.
2.7. Statistical analysis
In each analysis, three replications were performed for each concentration and negative control. The percent inhibition of each concentration for all experiments was statistically tested against the negative control using a one-tailed t-test with the following p-values: 0.12 (ns), 0.033 (*), 0.002 (**), and 0.001 (***). Pearson correlation was then used to analyze the linear relationship of each dependent variable, where R2 ≈ 1 indicates strong relation. All statistical analysis was carried out by Prism (GraphPad 9.3.1) software.
2.8. In silico study
2.8.1. Compound library construction and physicochemical property prediction
A library of 20 previously documented phytochemicals extracted from P. embelica using mass spectrometry was constructed after a comprehensive literature analysis. Swiss-ADME and pkCSM servers were employed to determine physicochemical properties and Absorption, Distribution, Metabolism, Excretion, Toxicity (ADMET) (Pires et al., 2015, Daina et al., 2017, Morshed et al., 2022, Jabin et al., 2023).
2.8.2. Retrieval and preparation of target proteins
15-LOXB (PDB ID:4NRE) was retrieved from Protein Data Bank (PDB). On the other hand, 15-LOXA was constructed from its fasta sequence (Accession no:P16050.3) through homology modelling server ‘HHpred’ on default settings (Söding et al., 2005). Following that, Procheck server was used to validate the constructed 3D structure of 15-LOXA with Ramachandran plot (Laskowski et al., 2006).
2.8.3. Molecular docking study
UCSF Chimera used for molecular optimization of ligands and target proteins (15-LOXA and 15-LOXB) (Pettersen et al., 2004). Afterward, molecular docking was performed using PyRx (version-0.8) to predict binding affinity of compounds with active sites of the structures of 15-LOXB and 15-LOXA (Arefin et al., 2021, Dey et al., 2022). After molecular interaction analysis, hydrogen bond and hydrophobic interactions were visualized using LigPlot+ (version-2.2.4) (Biswas et al., 2022, Ferdausi et al., 2022, Hasan et al., 2022). A widely used nonsteroidal anti-inflammatory drug ibuprofen (PubChem CID-3672) was considered as standard (Bushra and Aslam 2010).
2.8.4. Molecular dynamic simulation (MDS)
The molecular dynamics simulation (100 ns) was undertaken to determine the stability of the protein–ligand complex through the “Desmond v3.6 Program”, which is run on a linux interface. RMSF, RMSD, protein–ligand contacts, intramolecular hydrogen bonds and radius of gyration (Rg) were used to measure the stability of the protein–ligand (P-L) complex structure (Khan et al., 2021, Abdullah et al., 2023, Andalib et al., 2023, Biswas et al., 2023).
3. Results
3.1. Determination of antioxidant activity
At the highest concentration (400 µg/ml) the scavenging capacity of the extract was 53.56%, whereas the ascorbic acid scavenging capacity was 72.47%. In DPPH assay, the extract exhibited significant (P < 0.001) antioxidant activity with an IC50 of 311.31 µg/mL compared to standard ascorbic acid (IC50 of 130.53 µg/mL). The sample also expressed antioxidant activity (P < 0.001) in reducing power assay with EC50 of 196.20 µg/ml whereas, the EC50 value of standard ascorbic acid was 33.83 µg/ml (Fig. 1).
Fig. 1.
Antioxidant assays of tested crude methanolic extract of P. emblica. (A) % inhibition of DPPH free radical (B) IC50 (μg/ml) value (Mean ± SD) of DPPH free radical scavenging assay;(c) % reduction in reducing power assay (D) EC50 (μg/ml) value (Mean ± SD) of reducing power assay;(*** indicates p < 0.001 compared with respect to control group).
3.2. Determination of phytochemical constitution
The methanolic extract of P. emblica expressed a potent amount of phenolic, flavonoid, and tannin content. The extract exhibited 663.53 mg and 337.5 mg of GAE/g dry extract of phenolic and tannin content respectively whereas 418.89 mg of QE/g dry extract of flavonoid was found (Fig. 2).
Fig. 2.
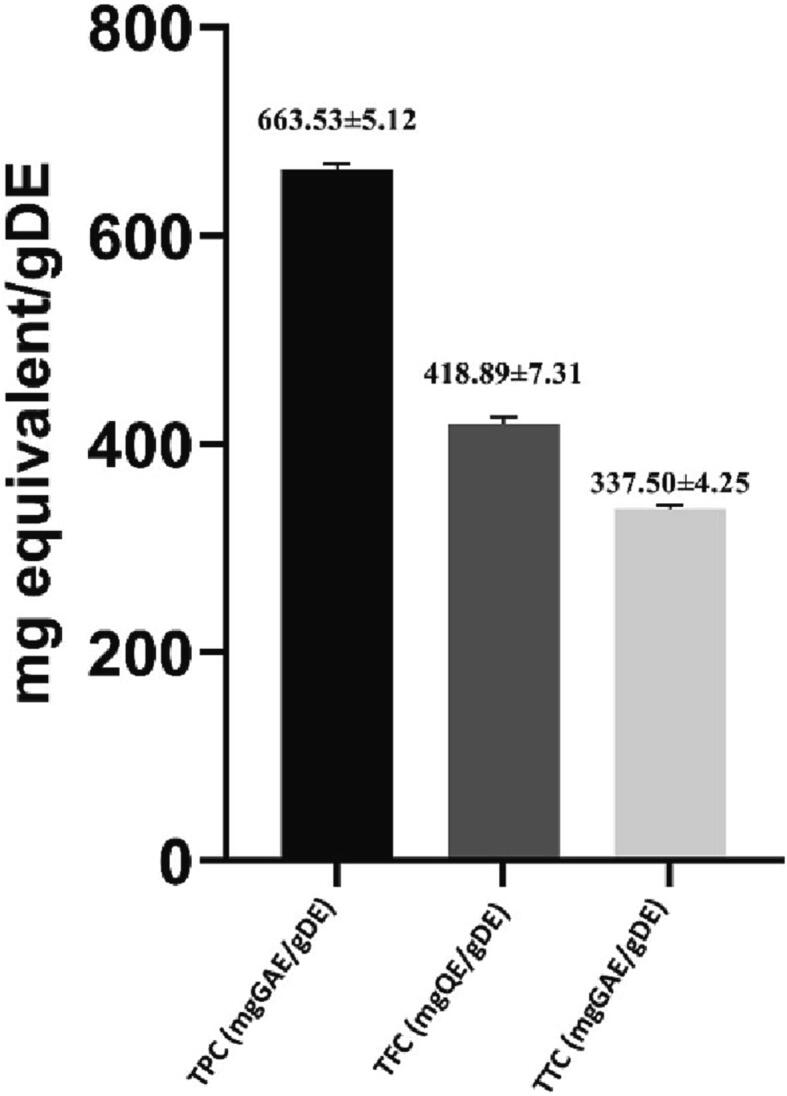
Quantitative analysis of phytochemical constitution (Mean ± SD) of crude extract of P. embilica.
3.3. Determination of in vitro anti-inflammatory activity
The 15-LOX inhibition assay demonstrated that P. emblica substantially inhibited the peroxidation of linoleic acid by soybean lipoxygenase in a dose-dependent manner. The methanolic extract of amla prominently inhibited 15-LOX with IC50 of 195.98 µg/mL compared to Quercetin with 19.62 µg/ml. In the case of hypotonicity-induced hemolysis assay, similar trends were observed where P. emblica extract exhibited inhibitory potential after the reference standard (Aspirin). IC50 value of extract was found 101.08 µg/ml while standard quercetin had the IC50 of 58.62 µg/ml (Fig. 3).
Fig. 3.
Anti-inflammatory assays of tested crude methanolic extract of P. embilica. (A) % inhibition of hemolysis in hypotonicity induced HRBCMS assay; (B) IC50 (μg/ml) value (Mean ± SD) of hypotonicity induced HRBCMS assay;(C) % inhibition of 15-LOX; (D) IC50 (μg/ml) value (Mean ± SD) of 15-LOX assay;(* indicates p < 0.033; ** indicates p < 0.002 and *** indicates p < 0.001 compared with respect to control group).
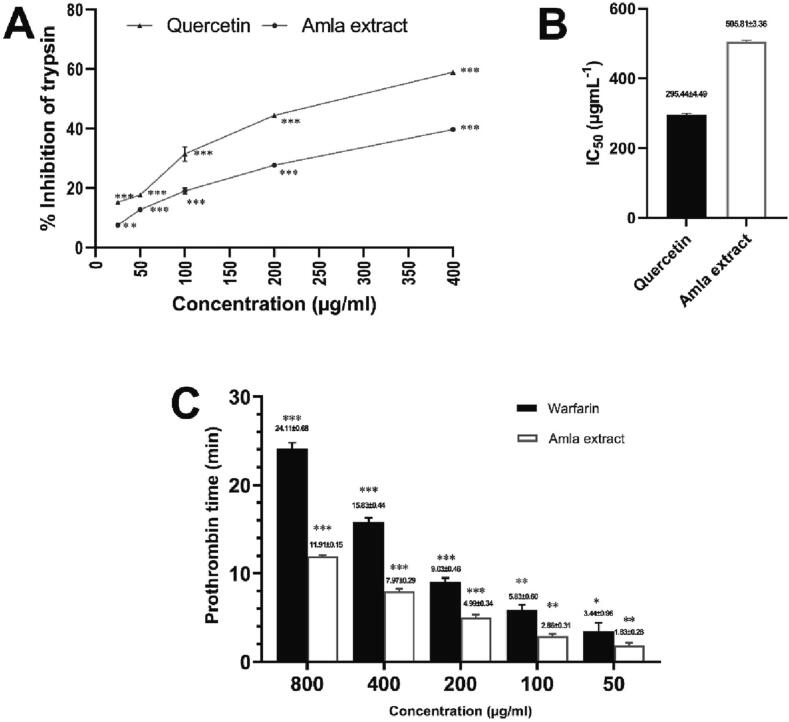
3.4. Determination of in vitro anti-coagulation activity
The serine protease inhibition and prothrombin time assay were conducted to determine the anti-coagulation activity of the sample. The extract inhibited trypsin with an IC50 of 505.81 µg/ml compared to standard quercetin (IC50 of 295.44 µg/ml). The prothrombin time assay indicated that clotting time was concentration-dependent for the sample tested. The longest time to clot was with standard warfarin (24.11 min at 800 μg/mL). At the concentration of 800 μg/mL, methanolic extract of Phyllanthus emblica required 11.91 min for full clotting (Fig. 4).
Fig. 4.
Anti-coagulation assays of tested crude methanolic extract of P. embilica. (A) % inhibition of trypsin in serine-protease inhibition assay (B) IC50 (μg/ml) value (Mean ± SD) of Serine protease inhibition assay; (C) Coagulation time of sample and standard in a dose-dependent manner;(* indicates p < 0.033; ** indicates p < 0.002 and *** indicates p < 0.001 compared with respect to control group).
3.5. Correlation analysis
A linear relationship was detected among antioxidant activity, anti-inflammatory, and anti-coagulation properties (Fig. 5). The R2 values for DPPH, reducing power, hemolysis, and serin protease assay range from 0.70 to 0.95, however 15-LOX inhibition and PTT assay did not correlate significantly. The correlation between the 15-LOX inhibition assay and the hemolysis assay was strong.
Fig. 5.
Correlation of antioxidant, anti-inflammatory and anticoagulation activity through Pearson square test, where R2 denotes Correlation coefficient.
3.6. In silico study
3.6.1. Structure evaluation of 15-LOXA through Ramachandran plot
If more than 90% of the amino acids are positioned in the most preferred area, the protein is considered to be well structured (Bilal et al., 2009). The generated 3D structure of 15-LOXA exhibited greater quality in the Ramachandran plot, with 534 (93.7 percent) residues in the most favorable positions (A, B, L) and the remaining 36 residues (6.3 percent) in additional permitted areas (a, b, l, p) (Fig. 6).
Fig. 6.
The 3D structure of 15-LOX A is represented in (A), and the Ramachandran plot is outlined in (B). In this plot, 93.7 percent of the amino acid residue was found in the most favored locations.
3.6.2. Physicochemical property analysis of reported compounds
All of the reported phytocompounds were tested for drug-likeness and toxicity. Table 1 displays the preferred phytochemicals along with their physicochemical and ADMET profiles.
Table 1.
ADMET Profiling of reported bio-active compound of P.emblica.
| Phytochemicals | P-CID | MW | #HA | NRB | HBA | HBD | TPSA | iLogP | BBB | IA(% Absorbed) | TC(log ml/min/kg) | LD50 (mol/kg) | HT | AT | NV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chebulagic Acid | CID: 442,674 | 954.66 | 68 | 5 | 27 | 13 | 447.09 | 0.42 | – | 95.705 | −2.64 | 2.48 | – | – | 3 |
| Gallic Acid | CID: 370 | 170.12 | 12 | 1 | 5 | 4 | 97.99 | 0.21 | – | 43.37 | 0.518 | 2.22 | – | – | 0 |
| Chebulinic Acid | CID: 72,284 | 956.68 | 68 | 12 | 27 | 13 | 447.09 | 0.95 | – | 34.33 | −2.11 | 2.5 | – | – | 3 |
| Corilagin | CID: 73,568 | 634.45 | 45 | 3 | 18 | 11 | 310.66 | 2.03 | – | 58.51 | 0.23 | 2.5 | – | – | 3 |
| Ellagic Acid | CID: 5,281,855 | 302.19 | 22 | 0 | 8 | 4 | 141.34 | 0.79 | – | 86.68 | 0.54 | 2.4 | – | – | 0 |
| Rutin | CID: 5,280,805 | 610.52 | 43 | 6 | 16 | 10 | 269.43 | 1.58 | – | 23.45 | −0.369 | 2.49 | – | – | 3 |
| Kaempferol | CID: 5,280,863 | 286.24 | 21 | 1 | 6 | 4 | 111.13 | 1.7 | – | 74.29 | 0.477 | 2.449 | – | – | 0 |
| Wogonin | CID: 5,281,703 | 284.26 | 21 | 2 | 5 | 2 | 79.9 | 2.55 | – | 92.68 | 0.294 | 2.265 | – | – | 0 |
| Epicatechin | CID: 72,276 | 290.27 | 21 | 1 | 6 | 5 | 110.34 | 1.47 | – | 68.83 | 0.183 | 2.428 | – | – | 0 |
| Catechin | CID: 9064 | 290.27 | 21 | 1 | 6 | 5 | 110.38 | 1.47 | – | 68.83 | 0.183 | 2.428 | – | – | 0 |
| Resveratrol | CID: 445,154 | 228.24 | 17 | 2 | 3 | 3 | 60.69 | 1.71 | + | 90.94 | 0.076 | 2.53 | – | + | 0 |
| Procyanidin B2 | CID: 122,738 | 578.52 | 42 | 3 | 12 | 10 | 220.76 | 1.35 | – | 66.75 | −0.085 | 2.482 | – | – | 3 |
| Myricetin | CID: 5,281,672 | 318.24 | 23 | 1 | 8 | 6 | 151.59 | 1.08 | – | 65.93 | 0.422 | 2.5 | – | – | 1 |
| Hamamelitannin | CID: 44,584,241 | 484.36 | 34 | 8 | 14 | 9 | 243.9 | 0.6 | – | 18.6 | 0.446 | 2.51 | – | – | 2 |
| Ethyl Gallate | CID: 13,250 | 198.17 | 14 | 3 | 5 | 3 | 86.99 | 1.21 | – | 76.7 | 0.674 | 1.94 | – | + | 0 |
| Methyl Gallate | CID: 7428 | 184.15 | 13 | 2 | 5 | 3 | 86.99 | 0.97 | – | 76.635 | 0.635 | 1.9 | – | + | 0 |
| Quercetin-3-O-Rhamnoside | CID: 5,280,459 | 448.38 | 32 | 3 | 11 | 7 | 190.28 | 1.6 | – | 52.71 | 0.364 | 2.586 | – | – | 2 |
| Ascorbic Acid | CID: 54,670,067 | 172.12 | 12 | 2 | 6 | 4 | 107.22 | −3.17 | – | 39.154 | 0.631 | 1.063 | – | – | 0 |
| Chlorogenic Acid | CID: 1,794,427 | 354.31 | 25 | 5 | 9 | 5 | 164.75 | 0.96 | – | 36.377 | 0.31 | 1.973 | – | – | 0 |
| Phyllanthin | CID: 358,901 | 418.52 | 30 | 13 | 6 | 0 | 55.38 | 4.26 | + | 98.349 | 0.591 | 2.325 | – | – | 0 |
P-CID = PubChem; CID MW = molecular weight (g/mol); #HA = number of heavy atoms; NRB = No. of rotatable bonds; HBA = hydrogen bond acceptor; HBD = hydrogen bond donor; TPSA = Topological polar surface area; iLogP = The n-octanol/water partition coefficient; BBB = Blood-Brain Barrier; IA = Intestinal absorption; TC = Total clearance (log ml/min/kg); LD50 = Oral rat acute toxicity; HT = Hepatotoxicity; AT = AMES toxicity; NV = No. of Lipinski’s rule violations.
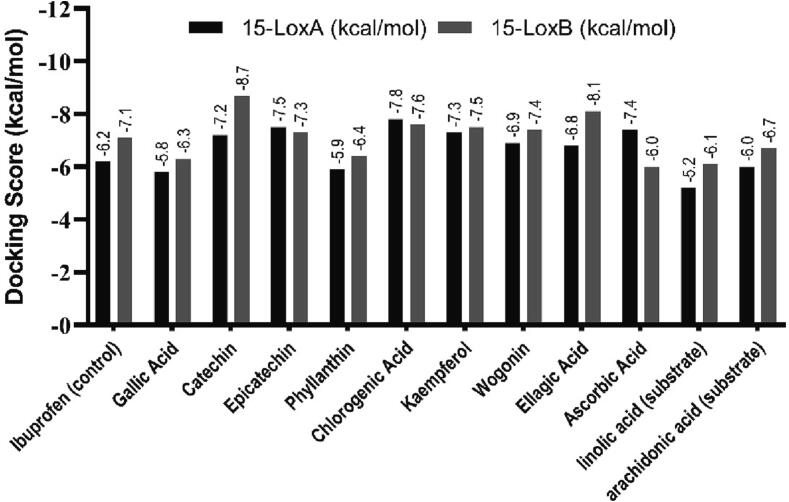
3.6.3. Molecular docking and post docking analysis
Following ADMET profiling 9 phytochemicals were selected for molecular interaction analysis. The docking score of selected phytochemicals was plotted in Fig. 6. Considering the docking score, hydrogen bonds and hydrophobic interaction with 15-LOXB, three compounds, Catechin (PubChem CID-9064), Chlorogenic acid (PubChem CID-1794427), and Ellagic acid (PubChem CID-5281855) were selected for further investigation (Fig. 8). Additionally, Epicatechin (PubChem CID-72276), Chlorogenic acid (PubChem CID-1794427), and Ascorbic acid (PubChem CID-54670067) demonstrated potential interactions with the 15-LOXA (Fig. 7).
Fig. 8.
Interaction analysis (3D) of 15-LOXA with Epicatechin (A), Chlorogenic acid (B), Ascorbic acid (C), control ibuprofen (D).
Fig. 7.
Docking score of selected phytochemicals.
The structural investigation of the P-L complex using Ligplot + revealed the protein and ligands interaction via hydrogen and hydrophobic interactions (Table 2) Fig. 10. The control ligand Ibuprofen was found to be stabilized by 3 respective hydrogen bonds for both 15-LOXA and 15-LOXB enzymes. On the other hand, Epicatechin showed 5 hydrogen bonds and Chlorogenic acid and Ascorbic acid exhibited 6 and 9 hydrogen bonds respectively against 15-LOXA. In addition, Catechin, Chlorogenic acid and Ellagic acid stabilized with 5,6 and 7 hydrogen bonds respectively against 15-LOXB (Fig. 9).
Table 2.
Analysis of the binding affinities of top candidate ligands with 15-lipoxygenase A and 15-lipoxygenase B compared to control ligand.
| Target porotein | Pubchem CID | Hydrogen bond | Hydrophobic interaction |
|---|---|---|---|
| 15-LOXB | Catechin | ASP625(2.96), ARG407(3.02), HIS394(2.87), TYR149(3.20), ARG145(3.05) | TYR107, GLN108, LEU389, ASN173, ILE174, ILE403 |
| Chlorogenic acid | ASN173(2.98), ARG145(2.80), GLN108(3.05,2.95), VAL117(2.82), GLN137(2.96) | ILE403, PHE399, TYR107, HIS394, MET148, GLU141, LEU116 | |
| Ellagic acid | ARG618(2.91), ARG407 (2.94, 2.98), TRP109 (2.97), ALA13 (3.20), GLU12 (3.12, 2.96) | ARG90, GLY11, ALA16, PHE88, ASP625 | |
| Ibuprofen | TYR408(3.15), LEU172(3.00), LYS175(2.88) | SER177, ALA13, ARG407, GLY11, GLU12, ARG90, PHE88, TRP109, ASN173 | |
| 15-LOXA | Epicatechin | GLU610(2.80), GLU609(3.03), TYR394(2.92), VAL601(2.93), LYS169(3.04) | HIS608, VAL603, ASN78, PHE76, TYR96, ARG393 |
| Chlorogenic acid | GLU609 (3.16), GLN599 (2.80), VAL601 (3.23), GLU656 (2.83, 2.71), SER658 (3.10) | HIS608, TYR394, VAL603, GLU610, LEU166, LYS169, MET602 | |
| Ascorbic acid | GLU609 (3.20), ASN78 (3.33, 2.84), ARG393 (3.13, 3.24, 2.96), TYR394 (2.95), HIS608 (2.99, 3.12) | GLU610, GLY10, LEU166, PHE76 | |
| Ibuprofen | ARG393 (2.98, 3.26), HIS608 (3.10) | LYS169, ASP168, TYR394, LEU166, GLU610, GLU609 |
Fig. 10.
2D interaction analysis using the Ligplot +. (A-D) shows the interaction between 15-LOXA and ligands where the indicators represent Epicatechin (A), Chlorogenic acid (B), Ascorbic acid (C), control ibuprofen (D). On the other hand, (E-H) shows the interaction of 15-LOXB with ligands where the indicators represent Catechin (E), Chlorogenic acid (F), Ellagic acid (G), control ibuprofen (H).
Fig. 9.
Interaction analysis (3D) of 15-LOXB with Catechin (A), Chlorogenic acid (B), Ellagic acid (C), control ibuprofen (D).
3.6.4. Molecular dynamic simulation
3.6.4.1. RMSD analysis
The root mean square deviation (RMSD) of a P-L complex enables the determination of the mean distance caused by complex dislocation from specific frame over a specified time period. It is quite acceptable to have a change in mean or average value of 1–3 Å or 0.1–0.3 nm between frames, but a change outside of this range suggests a major conformational shift in the protein (Sinha and Wang 2020). The RMSD was found by comparing the structures of the three compounds: catechin (blue), chlorogenic acid (red), and ellagic acid (green) complexed with the protein 15-LOXB, as shown in Fig. 12 (a). All of the compounds had RMSDs between 0.9 Å and 2.8 Å, compared to control Ibuprofen. Similarly, the RMSD of the compounds epicatechin (blue), chlorogenic acid (red), ascorbic acid (green), and control ibuprofen (violate) with the protein 15-LOXA has been calculated (Fig. 11 (a)) where Epicatechin and Ascorbic acid showed considerable fluctuation ranging from 1.2 to 3.3 Å. On the other hand, Chlorogenic acid had higher RMSD up to 4.2 Å with a median value of 3.6 Å.
Fig. 12.
RMSD (a), RMSF (b) and Rg (c) value of 100 ns MDS evaluations of target 15-LOXB complexed with three compounds catechin (A), chlorogenic acid (B), ellagic acid (C) and control ibuprofen (D).
Fig. 11.
RMSD (a), RMSF (b) and Rg (c) value of 100 ns MDS evaluations of target 15-LOXA complexed with three compounds epicatechin (A), chlorogenic acid (B), ascorbic acid (C) and control ibuprofen (D).
3.6.4.2. RMSF analysis
Root mean square fluctuation (RMSF) is a measure of how much a protein amino acid residue has fluctuated from a standard position over time. This is important for characterizing proteins since it may be used to determine the flexibility and fluctuation of the residues in the course of the simulation (Sinha and Wang 2020). The RMSF values of the catechin (blue), chlorogenic acid (Red), ellagic acid (green) and control ibuprofen (violate), in complex with the 15-LOXB, were ranged between 0.40 Å and 7.2 Å, as shown in Fig. 12-b. Chlorogenic acid showed higher RMSF with a and average 0.99 Å. Additionally, the RMSF of 15-LOXA in respect to selected compounds epicatechin (blue), chlorogenic acid (red), ascorbic acid (green), and control ibuprofen (violate) had shown fluctuation up to 5.66 Å (Fig. 11-b).
3.6.4.3. The radius of gyration (Rg) analysis
The radius of gyration (Rg) of an interacting complex can be defined as the configuration of its atoms around its axis. The calculation of Rg is one of the most important indicators to look for when anticipating a macromolecule's structural functioning because it shows variations in complex compactness across the simulation time (Sinha and Wang 2020). As a result, as shown in the Fig. 12(c), catechin (blue), chlorogenic acid (red), ellagic acid (green) and ibuprofen (violate) in interaction with the targeted protein 15-LOXB were studied in terms of Rg throughout a 100-ns simulation duration. All the compounds showed greater compactness, where Chlorogenic acid had the highest Rg value with an average of 4.64 Å. Similarly, epicatechin (blue), chlorogenic acid (red), ascorbic acid (green), and control ibuprofen (violate) complexed with 15-LOXA showed Rg value ranged from 2.3 to 4.3 Å, delineated in Fig. 11-c, indicating that the protein's binding site does not undergo major structural changes when the ligand compounds are bound (Javaid et al., 2018).
3.6.4.4. Analysis of intramolecular bonds
Over the course of the 100 ns simulation duration, the protein in complex with the specified ligands and their intramolecular interactions have been investigated using the “Simulation Interactions Diagram (SID)”. These interactions (or ‘contacts’) of the compounds, and control with 15-LOXB (PDB ID: 4NRE), and with 15-LOXA has been described, and shown in Fig. 13(a) and Fig. 13(b) respectively. It was shown that all compounds generated multiple connections via hydrogen, hydrophobic, ionic, and Water Bridge bonding and maintained these connections until the simulation finished, facilitating the creation of a stable binding with the targeted proteins.
Fig. 13.
Stacked bar chart of intramolecular interactions of target proteins with their respective ligands throughout the 100 ns simulation. The ligand-interaction with 15-LOXB is depicted in (a), where the indications represent epicatechin (a-A), chlorogenic acid (a-B), ascorbic acid (a-C) and control ibuprofen (a-D). On the other hand, the interactions of 15-LOXA with associated ligands are depicted in (b), where the indications represent catechin (b-A), chlorogenic acid (b-B), ellagic acid (b-C) and control ibuprofen (b-D).
4. Discussion
Under healthy conditions, oxidation and the generation of free radicals are normal phenomena, but when body cells are overrun by ROS, that leads to chronic inflammation, coagulation, and many other diseases. Nonsteroidal anti-inflammatory medicines (NSAIDs) and synthetic antioxidants are commonly recommended to treat oxidative damage and inflammation, but they have possible side effects and interact with other therapies (Lazzaroni and Bianchi Porro 2004). Plant derived bioactive substances could be a promising alternative and safe treatment agent for oxidative and inflammatory illnesses (Poulsen et al., 2020). As a result, the methanolic extract of Amla was studied in this study as a possible source of antioxidation, anticoagulant, and anti-inflammatory potential. Organic polar solvents such as methanol are commonly employed to extract highly polar bioactive compounds like phenolics and flavonoids (Chaves et al., 2020). Several investigations into methanolic extract of amla have been conducted to evaluate its bioactive properties, and each study has shown that methanolic extract exhibits substantial phenolic and flavonoid content (Liu et al., 2008, Liu et al., 2008, Yang and Liu, 2014, Sheoran et al., 2019).
Both the DPPH and reducing power assay certify antioxidant presence in the test extract compared to the standard. Several studies on Amla that used different assays to evaluate the antioxidant capacity also concluded that Amla is a potent source of antioxidants even much more than vitamin C (Khopde et al., 2001, Somasekhar et al., 2016). This study identified a substantial quantity of phenols, flavonoids, and tannin compounds. Some previous studies with different solvent extraction also validated the presence of a significant amount of phenolics in Amla extracts (Kumar et al., 2017, Li et al., 2019, Luan et al., 2019). The anti-inflammatory effects of the extracts were determined using two distinct approaches in our investigation. At a relatively low IC50 value, the methanolic extract was found to have the greatest capacity to protect RBC lysis against hyposaline compared to standard Quercetin. The anti-inflammatory activity of Amla has also been identified by previous studies using different extraction systems (Li et al., 2020). Several investigations in rodents used carrageenan to stimulate the cyclooxygenase pathway, where amla extract displayed substantial anti-inflammatory effect (Ihantola-Vormisto et al., 1997, Golechha et al., 2014). However, no research has been undertaken on the inhibitory activity of amla fruit in the 15-LOX pathway. So, in this study, anti-15 LOX activity was assessed through in vitro and in silico approaches.
The potential anticoagulation activity was validated by prothrombin time (PTT) assay and serine protease inhibition assay. While both assays substantiated a significant amount of anticoagulant in a concentration dependent manner compared to standard. Throughout the study, the negative control and test sample data were statistically evaluated by t-test to determine the level of significance of extract in each concentration of every experiment. All p-values<0.033 validated that the variance between group means of control and test samples are statistically significant. The R squared value from correlation analysis, being close to + 1, demonstrated that there is a strong linear correlation among antioxidant, anticoagulation and anti-inflammatory activity of the test sample. Some research evidently designated that polyphenols and polyphenol-rich extracts inhibit oxidation as well as show anticoagulant and anti-inflammatory properties simultaneously (Bijak et al., 2014, Yahfoufi et al., 2018). The antioxidant, anti-inflammatory and anticoagulant characteristics of phenolic compounds are well known for their ability to cause proton loss, chelate formation, and radical neutralization, among other qualities (Bijak et al., 2014, Yahfoufi et al., 2018, Hernandez-Ledesma and Martinez-Villaluenga, 2021). These phenolic compounds in amla extract, that was validated by the significant amount of total phenolic content, played a vital role in antioxidant, anti-inflammatory and anticoagulation capacity of Amla extract.
The in vitro assay was conducted using soybean 15-LOX, a prototype mimicking mammalian lipoxygenase, to evaluate the anti-inflammatory potential of amla crude extract. Then, employing documented phytochemicals from amla, in-silico docking and MDS were conducted against human 15-LOX to validate the anti-inflammatory potentials in humans. Additionally, all of the selected phytochemicals demonstrated higher binding efficacy than LOXs substrates linolic and arachidonic acid in docking studies against 15-LOXB and 15-LOXA. In molecular dynamic simulation of the P-L complex, chlorogenic acid was less stable than other compounds. Epicatechin and ascorbic acid exhibited greater compactness and stability with 15-LOXA, whereas catechin and ellagic acid were stable in contact with 15-LOXB. Therefore, on the basis of molecular docking and molecular dynamic simulation studies, epicatechin and catechin were identified as lead compounds to block 15-LOXA and 15-LOXB respectively. Many studies have confirmed our results that catechin and epicatechin have potent anti-inflammatory properties (Kürbitz et al., 2011, Stohs and Bagchi, 2015, Prakash et al., 2019).
In the in vitro studies (15-LOX inhibition assay and serine protease inhibition assay), it has been found that the crude amla extract substantially inhibited the 15-LOX enzyme (potential inflammatory mediator) and serine protease enzyme, which indicates the bioactive compounds in the extract can act as anti-inflammatory agents against the 15-LOX-mediated inflammopathies. Furthermore, in silico molecular docking against human 15-LOXs and MDS analysis were performed to validate the anti-inflammatory potential of amla. These in vitro and in silico studies support that amla-containing phytochemicals possess significant anti-inflammatory potential by effectively blocking 15-LOX, thereby preventing the pathological conditions regulated by 15-LOX.
5. Conclusion
The current study clearly showed that methanolic extract of amla exhibited possible antioxidant, anti-inflammatory, and anti-coagulation characteristics, notably validating the use of amla fruit in traditional medicine to treat inflammation and coagulation. Furthermore, this result implies the identification and isolation of specific compounds responsible for those activities using mass spectrometry and trail in vitro and in vivo for further investigation.
Author contribution
MA Sharif and AM Khan designed the methodology, conducted experiments, prepared visuals and wrote the initial draft; MH Rahman, S Bibi, P Biswas and MN Hasan assisted in the computational section of the study; R Salekeen and SM Nishan conducted formal analysis, A. Alshammari, M Alharbi and A Hayee validated the data, reviewed and edited the manuscript; ME Islam, KMD Islam and S Bibi, conceptualized and supervised the project. All authors discussed the results and agreed on the final manuscript.
Funding
Khulna University Research Cell, Khulna University, Bangladesh (grant number: KURC-RGP-11/2019) supported this research.
Authors are thankful to the Researchers Supporting Project number (RSP2023R491), King Saud University, Riyadh, Saudi Arabia.
Ethical statement
Khulna University Research Cell (ethical code KUAEC-2017/08/14) guidelines were strictly followed during the human blood collection process (for prothrombin timing and hypotonicity induced human red blood cell membrane stabilization assay).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors would like to express their utmost gratitude to Mr. Kumar Shubhro and Prof. Md. Morsaline Billah from Biotechnology and Genetic Engineering Discipline, Khulna University for their technical and intellectual assistance throughout the duration of this study.
Authors are thankful to the Khulna University Research Cell and the Researchers Supporting Project number (RSP2023R491), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Md. Arman Sharif, Email: arman.sharif10@gmail.com.
Arman Mahmud Khan, Email: mahmudarman0@gmail.com.
Rahagir Salekeen, Email: salekeenrahagir@gmail.com.
Md. Hafijur Rahman, Email: hafijurrahman.hr10@gmail.com.
Sakib Mahmud, Email: sakibnishan@gmail.com.
Shabana Bibi, Email: shabana.bibi.stmu@gmail.com.
Partha Biswas, Email: partha_160626@just.edu.bd.
Md. Nazmul Hasan, Email: mn.hasan@just.edu.bd.
Kazi Mohammed Didarul Islam, Email: didar950718@yahoo.com.
S.M. Mahbubur Rahman, Email: manmr2018@gmail.com.
Md. Emdadul Islam, Email: emdad@bge.ku.ac.bd.
Abdulrahman Alshammari, Email: abdalshammari@ksu.edu.sa.
Metab Alharbi, Email: mesalharbi@ksu.edu.sa.
Abdul Hayee, Email: khiljia.hayee@gmail.com.
References
- Abdullah, A., P. Biswas, M. Sahabuddin, et al., 2023. Molecular Dynamics Simulation and Pharmacoinformatic Integrated Analysis of Bioactive Phytochemicals from Azadirachta indica (Neem) to Treat Diabetes Mellitus. Journal of Chemistry. 2023,
- Afrin S., Pervin R., Sabrin F., et al. In vitro antioxidant activity, antimicrobial and preliminary cytotoxic activity of Cynometra ramiflora-a mangrove plant. J. Microbiol. Biotechnol. Food Sci. 2021;2021:844–850. [Google Scholar]
- Andalib K.M.S., Biswas P., Sakib M.R., et al. Identification of novel MCM2 inhibitors from Catharanthus roseus by pharmacoinformatics, molecular docking and molecular dynamics simulation-based evaluation. Inf. Med. Unlocked. 2023;39 doi: 10.1016/j.imu.2023.101251. [DOI] [Google Scholar]
- Arefin A., Ismail Ema T., Islam T., et al. Target specificity of selective bioactive compounds in blocking α-dystroglycan receptor to suppress Lassa virus infection: an in silico approach. J. Biomed. Res. 2021;35:459–473. doi: 10.7555/jbr.35.20210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijak M., Ziewiecki R., Saluk J., et al. Thrombin inhibitory activity of some polyphenolic compounds. Med. Chem. Res. 2014;23:2324–2337. doi: 10.1007/s00044-013-0829-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilal S., Iqbal H., Anjum F., et al. Prediction of 3D structure of P2RY5 gene and its mutants via comparative homology modelling. J. Comput. Biol. Bioinforma. Res. 2009;1:11–16. [Google Scholar]
- Birben E., Sahiner U.M., Sackesen C., et al. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas P., Dey D., Rahman A., et al. Analysis of SYK gene as a prognostic biomarker and suggested potential bioactive phytochemicals as an alternative therapeutic option for colorectal cancer: An in-silico pharmaco-informatics investigation. J. Pers. Med. 2021;11 doi: 10.3390/jpm11090888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas P., Hany Rumi O., Ahmed Khan D., et al. Evaluation of melongosides as potential inhibitors of NS2B-NS3 activator-protease of dengue virus (Serotype 2) by using molecular docking and dynamics simulation approach. J. Trop. Med. 2022;2022:7111786. doi: 10.1155/2022/7111786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas P., Bibi S., Yousafi Q., et al. Study of MDM2 as prognostic biomarker in brain-LGG cancer and bioactive phytochemicals inhibit the p53-MDM2 pathway: A computational drug development approach. Molecules. 2023;28 doi: 10.3390/molecules28072977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M.-E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995;28:25–30. [Google Scholar]
- Bushra R., Aslam N. An overview of clinical pharmacology of Ibuprofen. Oman Med. J. 2010;25:155–1661. doi: 10.5001/omj.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves J.O., de Souza M.C., da Silva L.C., et al. Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 2020;8 doi: 10.3389/fchem.2020.507887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daina A., Michielin O., Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey D., Biswas P., Paul P., et al. Natural flavonoids effectively block the CD81 receptor of hepatocytes and inhibit HCV infection: a computational drug development approach. Mol. Divers. 2022 doi: 10.1007/s11030-022-10491-9. [DOI] [PubMed] [Google Scholar]
- Dey D., Hasan M.M., Biswas P., et al. Investigating the anticancer potential of salvicine as a modulator of topoisomerase II and ROS signaling cascade. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.899009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey D., Hossain R., Biswas P., et al. Amentoflavone derivatives significantly act towards the main protease (3CL(PRO)/M(PRO)) of SARS-CoV-2: in silico admet profiling, molecular docking, molecular dynamics simulation, network pharmacology. Mol. Divers. 2022;1–15 doi: 10.1007/s11030-022-10459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftheriadis N., Poelman H., Leus N.G.J., et al. Design of a novel thiophene inhibitor of 15-lipoxygenase-1 with both anti-inflammatory and neuroprotective properties. Eur. J. Med. Chem. 2016;122:786–801. doi: 10.1016/j.ejmech.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdausi N., Islam S., Rimti F.H., et al. Point-specific interactions of isovitexin with the neighboring amino acid residues of the hACE2 receptor as a targeted therapeutic agent in suppressing the SARS-CoV-2 influx mechanism. J. Adv. Vet. Anim. Res. 2022;9:230–240. doi: 10.5455/javar.2022.i588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaire B.P., Subedi L. Phytochemistry, pharmacology and medicinal properties of Phyllanthus emblica Linn. Chin. J. Integr. Med. 2014 doi: 10.1007/s11655-014-1984-2. [DOI] [PubMed] [Google Scholar]
- Golechha M., Sarangal V., Ojha S., et al. Anti-inflammatory effect of emblica officinalis in rodent models of acute and chronic inflammation: Involvement of possible mechanisms. Int. J. Inflam. 2014;2014 doi: 10.1155/2014/178408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A., Biswas P., Bondhon T.A., et al. Can Artemisia herba-alba be useful for managing COVID-19 and comorbidities? Molecules. 2022;27 doi: 10.3390/molecules27020492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M.M., Zilani M.N.H., Akter S., et al. UHPLC-Q/Orbitrap/MS based chemical fingerprinting and hepatoprotective potential of a medicinal plant, Morinda angustifolia Roxb. S. Afr. J. Bot. 2022;148:561–572. doi: 10.1016/j.sajb.2022.05.037. [DOI] [Google Scholar]
- Hernandez-Ledesma B., Martinez-Villaluenga C. Academic Press; 2021. Current advances for development of functional foods modulating inflammation and oxidative stress. [Google Scholar]
- Ihantola-Vormisto A., Summanen J., Kankaanranta H., et al. Anti-inflammatory activity of extracts from leaves of Phyllanthus emblica. Planta Med. 1997;63:518–524. doi: 10.1055/s-2006-957754. [DOI] [PubMed] [Google Scholar]
- Islam M.E., Islam K.M.D., Billah M.M., et al. Antioxidant and anti-inflammatory activity of Heritiera fomes (Buch.-Ham), a mangrove plant of the Sundarbans. Adv. Trad. Med. 2020;20:189–197. [Google Scholar]
- Islam M.A., Zilani M.N.H., Biswas P., et al. Evaluation of in vitro and in silico anti-inflammatory potential of some selected medicinal plants of Bangladesh against cyclooxygenase-II enzyme. J. Ethnopharmacol. 2022;285 doi: 10.1016/j.jep.2021.114900. [DOI] [PubMed] [Google Scholar]
- Jabin A., Uddin M.F., Al Azad S., et al. Target-specificity of different amyrin subunits in impeding HCV influx mechanism inside the human cells considering the quantum tunnel profiles and molecular strings of the CD81 receptor: a combined in silico and in vivo study. In Silico Pharmacol. 2023;11:8. doi: 10.1007/s40203-023-00144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaid S., Naz S., Amin I., et al. Computational and biological characterization of fusion proteins of two insecticidal proteins for control of insect pests. Sci. Rep. 2018;8:4837. doi: 10.1038/s41598-018-23138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabel A.M. Free radicals and antioxidants: role of enzymes and nutrition. World J. Nutr. Health. 2014;2:35–38. [Google Scholar]
- Khan R.A., Hossain R., Siyadatpanah A., et al. Diterpenes/diterpenoids and their derivatives as potential bioactive leads against dengue virus: A computational and network pharmacology study. Molecules. 2021;26 doi: 10.3390/molecules26226821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A.M., Sharif M.A., Salekeen R., et al. In vitro and in silico investigation of garlic’s (Allium sativum) bioactivity against 15-lipoxygenase mediated inflammopathies. J. Herbmed Pharmacol. 2023;12:283–298. [Google Scholar]
- Khopde S., Priyadarsini K.I., Mohan H., et al. Characterizing the antioxidant activity of amla (Phyllanthus emblica) extract. Curr. Sci. 2001:185–190. [Google Scholar]
- Kumar S., Singh A., Kumar B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017;7:214–222. doi: 10.1016/j.jpha.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kürbitz C., Heise D., Redmer T., et al. Epicatechin gallate and catechin gallate are superior to epigallocatechin gallate in growth suppression and anti-inflammatory activities in pancreatic tumor cells. Cancer Sci. 2011;102:728–734. doi: 10.1111/j.1349-7006.2011.01870.x. [DOI] [PubMed] [Google Scholar]
- Laskowski, R., M. MacArthur and J. Thornton, 2006. PROCHECK: validation of protein-structure coordinates.
- Lazzaroni M., Bianchi Porro G. Gastrointestinal side-effects of traditional non-steroidal anti-inflammatory drugs and new formulations. Aliment. Pharmacol. Ther. 2004;20(Suppl 2):48–58. doi: 10.1111/j.1365-2036.2004.02037.x. [DOI] [PubMed] [Google Scholar]
- Li Y., Guo B., Wang W., et al. Characterization of phenolic compounds from Phyllanthus emblica fruits using HPLC-ESI-TOF-MS as affected by an optimized microwave-assisted extraction. Int. J. Food Prop. 2019;22:330–342. [Google Scholar]
- Li P.-H., Wang C.-W., Lu W.-C., et al. Antioxidant, anti-inflammatory activities, and neuroprotective behaviors of Phyllanthus emblica L. fruit extracts. Agriculture. 2022;12:588. [Google Scholar]
- Li W., Zhu H.W., Chen Y.J., et al. Bioactivity-guided isolation of anti-inflammatory components from Phyllanthus emblica. Food Sci. Nutr. 2020;8:2670–2679. doi: 10.1002/fsn3.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Cui C., Zhao M., et al. Identification of phenolics in the fruit of emblica (Phyllanthus emblica L.) and their antioxidant activities. Food Chem. 2008;109:909–915. doi: 10.1016/j.foodchem.2008.01.071. [DOI] [PubMed] [Google Scholar]
- Liu X., Zhao M., Wang J., et al. Antioxidant activity of methanolic extract of emblica fruit (Phyllanthus emblica L.) from six regions in China. J. Food Compos. Anal. 2008;21:219–228. [Google Scholar]
- Lopes-Pires M.E., Frade-Guanaes J.O., Quinlan G.J. Clotting dysfunction in sepsis: A role for ROS and potential for therapeutic intervention. Antioxidants (Basel) 2021;11 doi: 10.3390/antiox11010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan Y., Luan Y., Zhao Y., et al. Isorhamnetin in Tsoong blocks Hsp70 expression to promote apoptosis of colon cancer cells. Saudi J. Biol. Sci. 2019;26:1011–1022. doi: 10.1016/j.sjbs.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshed A., Al Azad S., Mia M.A.R., et al. Oncoinformatic screening of the gene clusters involved in the HER2-positive breast cancer formation along with the in silico pharmacodynamic profiling of selective long-chain omega-3 fatty acids as the metastatic antagonists. Mol. Divers. 2022 doi: 10.1007/s11030-022-10573-8. [DOI] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pham A.T., Malterud K.E., Paulsen B.S., et al. α-Glucosidase inhibition, 15-lipoxygenase inhibition, and brine shrimp toxicity of extracts and isolated compounds from Terminalia macroptera leaves. Pharm. Biol. 2014;52:1166–1169. doi: 10.3109/13880209.2014.880486. [DOI] [PubMed] [Google Scholar]
- Pires D.E., Blundell T.L., Ascher D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015;58:4066–4072. doi: 10.1021/acs.jmedchem.5b00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polshettiwar S., Ganjiwale R., Wadher S., et al. Spectrophotometric estimation of total tannins in some ayurvedic eye drops. Indian J. Pharm. Sci. 2007;69:574. [Google Scholar]
- Poulsen N.B., Lambert M.N.T., Jeppesen P.B. The effect of plant derived bioactive compounds on inflammation: A systematic review and meta-analysis. Mol. Nutr. Food Res. 2020;64:e2000473. doi: 10.1002/mnfr.202000473. [DOI] [PubMed] [Google Scholar]
- Prakash M., Basavaraj B., Murthy K.C. Biological functions of epicatechin: Plant cell to human cell health. J. Funct. Foods. 2019;52:14–24. [Google Scholar]
- Rahman M.A., Rahman M.D.H., Hossain M.S., et al. Molecular insights into the multifunctional role of natural compounds: autophagy modulation and cancer prevention. Biomedicines. 2020;8 doi: 10.3390/biomedicines8110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.A., Rahman M.H., Biswas P., et al. Potential therapeutic role of phytochemicals to mitigate mitochondrial dysfunctions in Alzheimer's disease. Antioxidants (Basel) 2020;10 doi: 10.3390/antiox10010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.S., Zilani M.N.H., Islam M.A., et al. In Vivo Neuropharmacological potential of Gomphandra tetrandra (Wall.) Sleumer and in-silico study against β-amyloid precursor protein. Processes. 2021;9:1449. [Google Scholar]
- Rebaya A., Belghith S.I., Baghdikian B., et al. Total phenolic, total flavonoid, tannin content, and antioxidant capacity of Halimium halimifolium (Cistaceae) J. Appl. Pharmaceut. Sci. 2015;5:052–057. [Google Scholar]
- Sheoran S., Nidhi P., Kumar V., et al. Altitudinal variation in gallic acid content in fruits of Phyllanthus emblica L. and its correlation with antioxidant and antimicrobial activity. Vegetos. 2019;32:387–396. [Google Scholar]
- Sinha S., Wang S.M. Classification of VUS and unclassified variants in BRCA1 BRCT repeats by molecular dynamics simulation. Comput. Struct. Biotechnol. J. 2020;18:723–736. doi: 10.1016/j.csbj.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söding J., Biegert A., Lupas A.N. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005;33:W244–W248. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohel M., Biswas P., Al Amin M., et al. Genistein, a potential phytochemical against breast cancer treatment-insight into the molecular mechanisms. Processes. 2022;10:415. [Google Scholar]
- Somasekhar V., Ashok P., Kameswari S.A.R., et al. Comparative antioxidant and bioavailability studies of Vitamin C in Phyllanthus emblica Linn. and its combinations with Piper nigrum Linn. and Zingiber officinale Roscoe. Braz. J. Pharm. Sci. 2016;52:35–43. [Google Scholar]
- Srivasuki K. Nutritional and health care benefits of Amla. J. Pharmacogn. 2012;3:147–151. [Google Scholar]
- Stohs S.J., Bagchi D. Antioxidant, anti-inflammatory, and chemoprotective properties of Acacia catechu heartwood extracts. Phytother. Res. 2015;29:818–824. doi: 10.1002/ptr.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal V.J., Fanburg B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- Umeno A., Biju V., Yoshida Y. In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer's disease, Parkinson's disease, and diabetes. Free Radic. Res. 2017;51:413–427. doi: 10.1080/10715762.2017.1315114. [DOI] [PubMed] [Google Scholar]
- Variya B.C., Bakrania A.K., Patel S.S. Emblica officinalis (Amla): A review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol. Res. 2016;111:180–200. doi: 10.1016/j.phrs.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Wisastra R., Dekker F.J. Inflammation, cancer and oxidative lipoxygenase activity are intimately linked. Cancers (Basel) 2014;6:1500–1521. doi: 10.3390/cancers6031500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahfoufi N., Alsadi N., Jambi M., et al. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10 doi: 10.3390/nu10111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Kortesniemi M., Liu P., et al. Analysis of hydrolyzable tannins and other phenolic compounds in emblic leafflower (Phyllanthus emblica L.) fruits by high performance liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2012;60:8672–8683. doi: 10.1021/jf302925v. [DOI] [PubMed] [Google Scholar]
- Yang B., Liu P. Composition and biological activities of hydrolyzable tannins of fruits of Phyllanthus emblica. J. Agric. Food Chem. 2014;62:529–541. doi: 10.1021/jf404703k. [DOI] [PubMed] [Google Scholar]
- Yesmin S., Paul A., Naz T., et al. Membrane stabilization as a mechanism of the anti-inflammatory activity of ethanolic root extract of Choi (Piper chaba) Clin. Phytosci. 2020;6:1–10. [Google Scholar]
- Zilani M.N.H., Islam M.A., Biswas P., et al. Metabolite profiling, anti-inflammatory, analgesic potentials of edible herb Colocasia gigantea and molecular docking study against COX-II enzyme. J. Ethnopharmacol. 2021;281 doi: 10.1016/j.jep.2021.114577. [DOI] [PubMed] [Google Scholar]