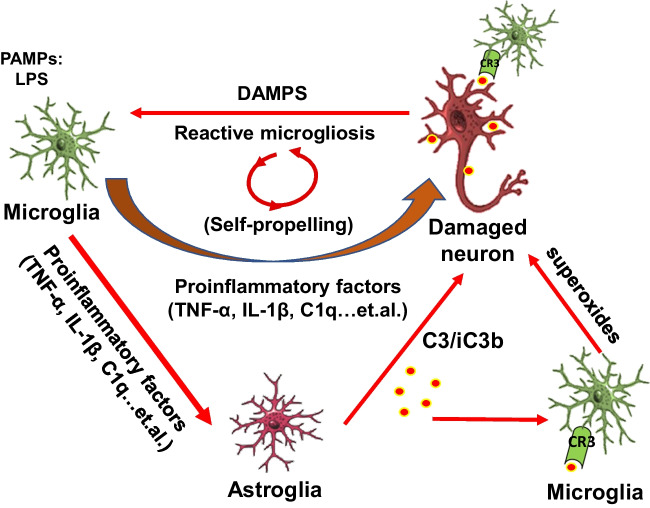
Fig. 9.
Schematic drawing showing how the possible interactions between neurons, astroglia, and microglia regulate the C3 expression and neurotoxic roles of C3 in maintaining the progression of chronic neuroinflammation and leading to neurodegeneration. During the acute phase, microglia activated by pathogen-associated molecular patterns (PAMPs), like LPS, can produce multiple proinflammatory factors to combat invading microorganisms but also can damage surrounding neurons. Proinflammatory factors released from activated microglia can also trigger astroglia to have delayed release of immune factors, including complement C3 and iC3b to label the damaged neurons during the chronic phase of neuroinflammation. The attached C3 can attract microglia to further damage neurons. Furthermore, C3 from astroglia and DAMPs from the damaged neurons or other cells can trigger the activation of the Mac1-NOX2 axis in microglia to further produce superoxides to cause oxidative stress and maintain the reactive microgliosis. During this chronic phase, microglia cease to produce proinflammatory cytokines but continue to enhance the production of C3, superoxide, and other delayed immune factors. Thus, sustaining activated microglia, astroglia, and damaged neurons can result in a self-propelling circle to maintain chronic inflammation and subsequent neurodegeneration