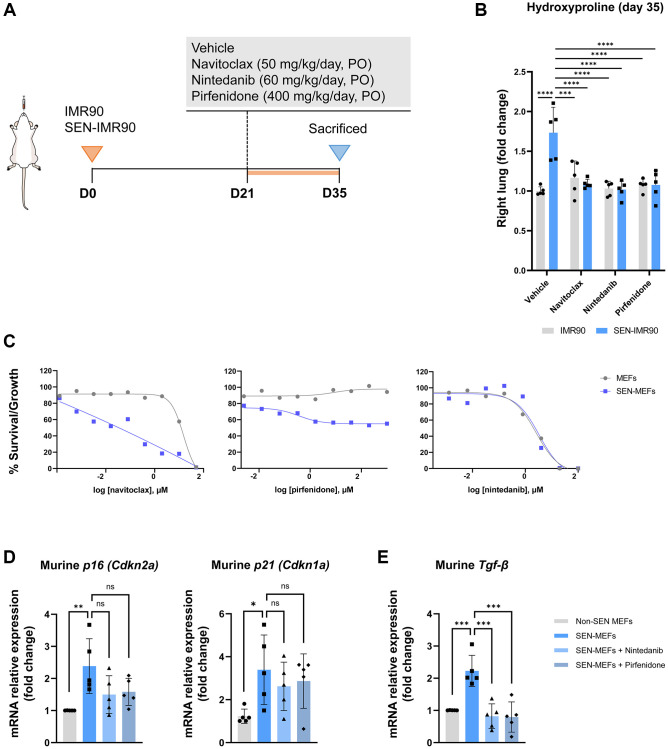
Figure 5.
Effects of antifibrotic and senolytic drugs. (A) Scheme showing the experimental design to assess the effect of antifibrotic or senolytic drugs. Nude mice were randomized after 21 days post-injection of irradiated SEN-IMR90 cells or IMR90 cells as negative control, to either the two approved antifibrotics drugs (nintedanib or pirfenidone), a senolytic drug (navitoclax), or vehicle, for two weeks. (B) Hydroxyproline content in the right lung tissues of mice treated with navitoclax, nintedanib or pirfenidone, compared with control; n = 5 each group. Statistical significance was assessed by the one-way ANOVA with Tukey test: ***p < 0.001; ****p < 0.0001. (C) Senolytic activity of navitoclax (left panel), pirfenidone (middle panel) or nintedanib (right panel). Diagram showing the senolytic activity of these agents after exposure of senescent MEFs (SEN-MEFs) or non-senescent MEFs (NS-MEFs) to increasing concentration of navitoclax, pirfenidone, nintedanib or vehicle for 72 hours, as confirmed by relative expression of the mRNA coding for murine senescence markers (Cdkn2a/p16INK4a and Cdkn1a/p21Cip1/Waf1), measured relative to Actin-b levels in lung cell extracts of nintedanib or pirfenidone group compared to control (D); n = 5 each group, independent experiments. Statistical significance was assessed by the one-way ANOVA with Tukey test: **p < 0.01; *p < 0.05. (E) Relative expression of the mRNA coding for Tgf-β (transforming growth factor-β) was measured relative to Actin-b levels in SEN-MEFs treated with pirfenidone or nintenadib, compared with control; n = 5 each group, independent experiments. Statistical significance was assessed by the one-way ANOVA with Tukey test: ***p < 0.001. For further explanations, see text.