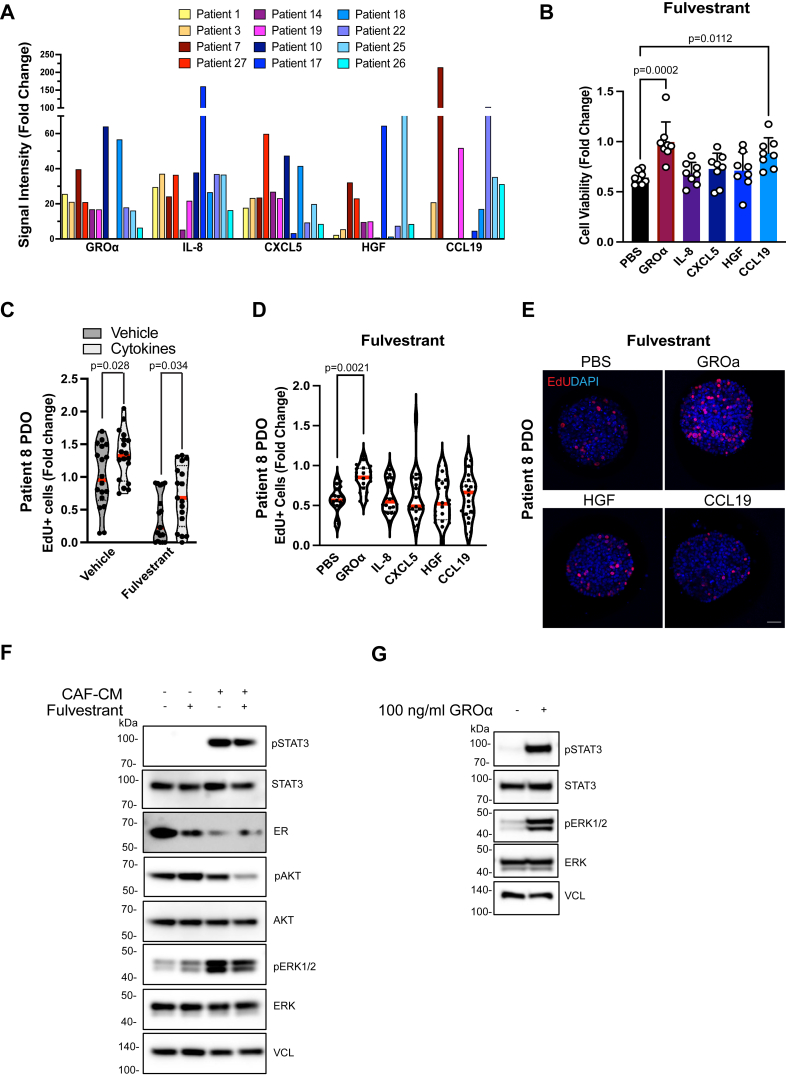
Figure 6.
CAF-secreted cytokines drive resistance to fulvestrant.A, quantification of the signal intensity of the five highly secreted cytokines from the cytokine arrays of CAF-CM (patients #1-27) normalized to cancer cell-CM. B, cell viability of MCF7 cells treated with recombinant human GROα/CXCL1, IL-8, CXCL5, HGF, CCL19, or PBS (100 ng/ml) and fulvestrant (500 nM). Presto Blue was used to assess cell viability after 72 h. Data are normalized to PBS treated groups and is a representative of three independent experiments. Error bars are SEM. C and D, quantification of EdU+ cells in PDOs treated with a cytokine cocktail and fulvestrant (500 nM) (C) or with recombinant human GROα, IL-8, CXCL5, HGF, CCL19, or PBS (100 ng/ml) and fulvestrant (500 nM) from two independent experiments. Proliferation was assessed by pulsing PDOs with EdU for 4 h and quantifying the ratio of EdU+ cells per total (DAPI+) number of cells in 20 PDOs. Red line indicates the mean. E, representative confocal images of PDOs treated with recombinant human GROα, HGF, CCL19, or PBS (100 ng/ml) and fulvestrant (500 nM). F, Western blot analysis of CAF-CM stimulated signaling pathway proteins pSTAT3, STAT3, ER, pAKT, AKT, pERK1/2, and ERK1/2 in PDOs grown in NC or CAF-CM and treated with 500 nM fulvestrant for 96 h. G, Western blot analysis of GROα stimulated signaling pathway proteins pSTAT3, STAT3, pERK1/2, and ERK1/2 in MCF7 cells starved overnight and incubated for 30 min with 100 ng/ml GROα. VCL was used as loading control. Student’s t test was used to determine significance by comparing each treatment to the PBS control in (B) and (D). The scale bar represents 40 μm. CAF, cancer-associated fibroblasts; DAPI, 4′,6-diamidino-2-phenylindole; EdU, 5-ethynyl-2′-deoxyuridine; ER, estrogen receptor; GROα, growth-regulated oncogene α; HGF, hepatocyte growth factor; IL, interleukins; PDOs, patient-derived organoids; VCL, vinculin.