Abstract
True thymic hyperplasia (TTH) is a rare cause of a fat-containing mediastinal mass, most commonly found in infants and young children. We report a case of TTH in a 22-year-old adult, successfully managed via minimally invasive video-assisted thoracoscopic surgery.
Keywords: True thymic hyperplasia, Video-assisted thoracoscopic surgery
Introduction
There are two morphological forms of thymic hyperplasia: true thymic hyperplasia (TTH) and lymphofollicular hyperplasia (LFH) [1]. True thymic hyperplasia is characterized by an increase in the size and weight of the thymic gland, with retention of the normal histological architecture [2]. This type of proliferation is unknown in any other organ [3]. Idiopathic TTH is an extremely rare phenomenon [2]. It does not occur in association with a provocative systemic stress or disease process, unlike other TTH subtypes. Patients with TTH may be asymptomatic, or present with symptoms related to compression of adjacent mediastinal structures. Histological examination is required for definitive diagnosis [4], with surgical resection required for the management of larger lesions [3].
Case report
A 22-year-old male, undergoing CT chest following a motor vehicle accident, was incidentally found to have a large (76 mm x 148 mm x 123 mm) fat and soft tissue attenuation anterior mediastinal mass (Fig. 1). On initial presentation, the patient was asymptomatic with a normal physical examination. A full blood count revealed a mild lymphocytosis which had been present for 8 years prior to the current presentation. The patient had no other known pre-existing medical history and no history of recent illness or systemic stress.
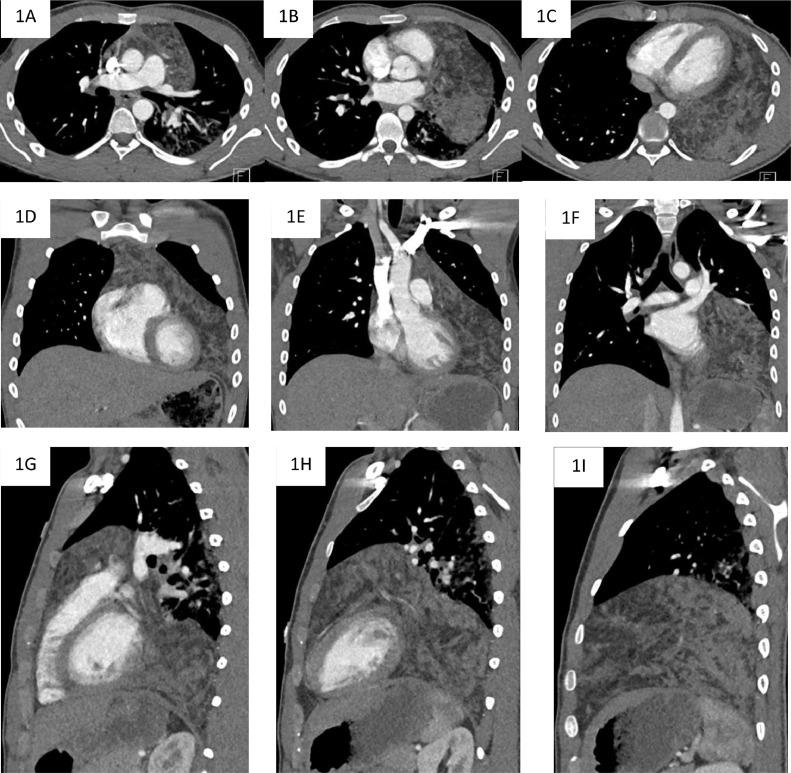
Fig. 1.
Selected axial (A–C), coronal (D–F), and sagittal (G–I) CT images demonstrating a large fat and soft tissue attenuation anterior mediastinal mass with extension into the left hemithorax.
On CT imaging, the mass abutted the visceral pleura, pericardium, great vessels, and diaphragm but did not demonstrate features of direct invasion into these structures. Due to the presence of bulk fat and the absence of aggressive imaging features, the lesion was radiologically favored to represent a thymolipoma.
Given a combination of factors including patient preference, favored benign etiology, young age, and large size of the lesion, VATS resection without biopsy was performed, with the lesion successfully dissected from adherent structures and removed in its entirety via a subxiphoid incision (Fig. 2). The patient recovered swiftly without complication and was discharged from the hospital on postoperative day 2.
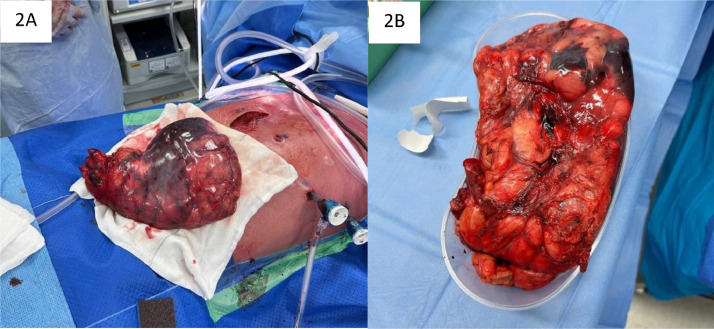
Fig. 2.
Intraoperative images of the mass following successful resection.
The resected gross specimen weighed 1092 grams. The histology findings (Fig. 3) and the immunohistochemical analysis (Fig. 4) were consistent with a diagnosis of TTH.
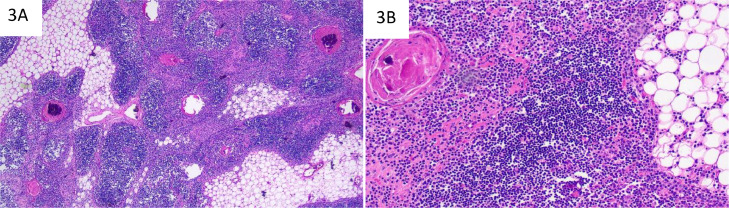
Fig. 3.
H&E stained sections. Islands of thymic tissue alternating with mature adipose tissue (A, low power). Well-delineated medullary zones with prominent Hassall's corpuscles; cortical zones demonstrate no lymphoid follicle formation. No evidence of morphologically distinct thymic tissue in the periphery (B, high power).
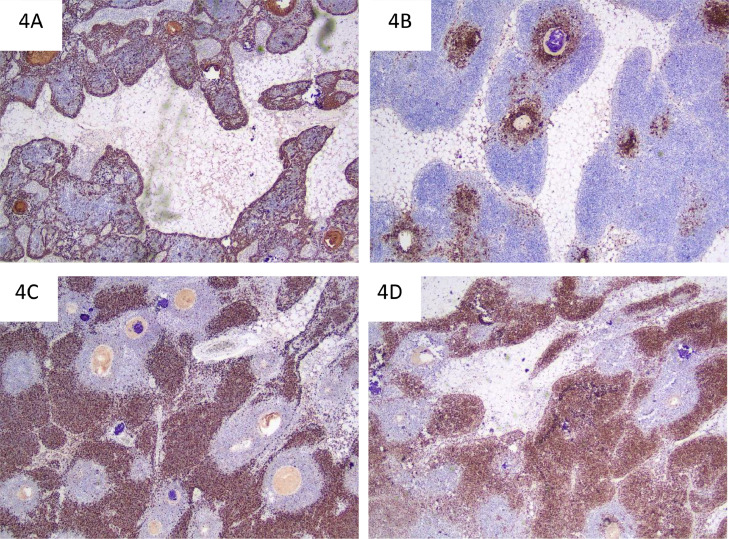
Fig. 4.
Immunoperoxidase stains. CK34BE12 (A) highlights thymic epithelial cells which are most numerous in the periphery of the thymic parenchymal islands and medullary zones. CD20 (B) highlights mature B lymphocytes in the medullary zones. Immature thymic T lymphocytes in the cortical zones are positive for TdT (C) and have a high Ki67 proliferative index (D).
Additional postoperative blood testing excluded common secondary causes of TTH such as thyrotoxicosis, Grave's disease, red cell aplasia, and acromegaly. There was no basis for preoperative lymphocytosis, although this has been described in some cases of TTH [5]. At discharge, lymphocyte count had reduced compared to preoperative levels.
Discussion
Principal differential considerations for a fat-containing anterior mediastinal mass in an adult include thymolipoma, teratoma, lipoma, liposarcoma, and Morgagni hernia [6]. Differentiation between benign and malignant mediastinal masses on imaging is often difficult and tissue diagnosis is invariably required to achieve diagnostic certainty. However, the potential for biopsy sampling error is an important consideration, particularly with large lesions containing bulk fat. Biopsy also risks compromising the integrity of the lesion/surgical tissue planes; this is of potential relevance if the lesion is found to be malignant [7].
Surgical resection is the primary treatment for TTH, with common techniques including VATS, posterolateral thoracotomy, and median sternotomy [3]. A left-sided VATS approach was selected due to the anatomical location of the lesion which facilitated excellent views of the thymic bed from the diaphragm to the neck and facilitated magnification of critical dissection plans. Additionally, there is evidence that VATS is associated with significantly improved short-term postoperative pulmonary function, compared to a typical median sternotomy approach [3].
Conclusion
True thymic hyperplasia is a potential differential diagnosis in an adult presenting with a large fat-containing mediastinal mass. When no secondary cause for true thymic hyperplasia is found, a diagnosis of idiopathic TTH remains a consideration in an adult. This lesion can be safely managed using minimally invasive surgical techniques such as VATS, with rapid postsurgical recovery and favorable prognosis.
Patient consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Hofmann WJ, Moller P, Otto HF. Thymic hyperplasia. I. True thymic hyperplasia. Review of the literature. Klin Wochenschr. 1987;65(2):49–52. doi: 10.1007/BF01745472. [DOI] [PubMed] [Google Scholar]
- 2.Levine GD, Rosai J. Thymic hyperplasia and neoplasia: a review of current concepts. Hum Pathol. 1978;9:495–515. doi: 10.1016/s0046-8177(78)80131-2. [DOI] [PubMed] [Google Scholar]
- 3.Linegar AG, Odell JA, Fennell WM, Close PM, De Groot MK, Casserly DR, et al. Massive thymic hyperplasia. Ann Thorac Surg. 1993;55:1197–1201. doi: 10.1016/0003-4975(93)90033-e. [DOI] [PubMed] [Google Scholar]
- 4.Regal MA. Gigantic enlargement of the thymus gland. Saudi Med J. 2007;28:1587–1589. [PubMed] [Google Scholar]
- 5.Ricci C, Pescarmona E, Rendina EA, Venuta F, Ruco LP, Baroni CD. True thymic hyperplasia: a clinicopathological study. Ann Thorac Surg. 1989;47(5):741–745. doi: 10.1016/0003-4975(89)90131-8. [DOI] [PubMed] [Google Scholar]
- 6.Quint LE. Imaging of anterior mediastinal masses. Cancer Imaging. 2007;7:S56–S62. doi: 10.1102/1470-7330.2007.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou D, Luo H, Feng Y, Zeng B, Lei Y. Massive thymic hyperplasia in an adult: a rare case report and literature review. Int J Surg Case Rep. 2018;47:104–108. doi: 10.1016/j.ijscr.2018.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]