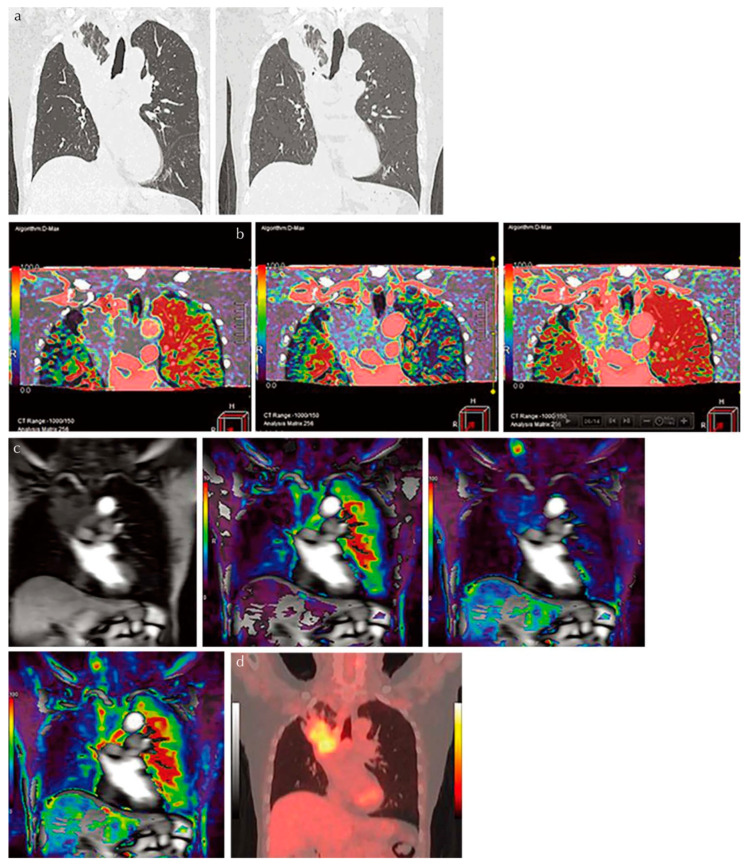
Figure 3.
An 81-year-old male patient with squamous cell carcinoma treated with chemoradiotherapy and assessed as NC. Progression-free and overall survivals at 15 and 24 months (permission from reference [22]). (a) Thin-section MPR image derived from thin-section CT data (L to R: MPR images obtained pre- and post-treatment at lung window setting) show lung cancer in the right upper lobe. This case was assessed as NC according to response evaluation criteria for solid tumors (RECIST ver.1.1). (b) Perfusion maps derived from dynamic first-pass CE-perfusion area-detector CT assessed with the dual-input maximum slope method (L to R: pulmonary arterial perfusion, systemic arterial perfusion, and total perfusion maps) for the same targeted lesion. Pulmonary arterial perfusion, systemic arterial perfusion, and total perfusion were 13.6, 18.9, and 32.5 mL/100 mL/min, respectively. This case was assessed as a RECIST-based non-responder for systemic arterial and total perfusions and as true positive. (c) Source image and perfusion maps obtained with dynamic first-pass CE-perfusion MR imaging assessed with the dual-input maximum slope method (L to R: source image, pulmonary arterial perfusion, systemic arterial perfusion, and total perfusion maps) for the same targeted lesion. Pulmonary arterial perfusion, systemic arterial perfusion, and total perfusion were 9.2, 28.9, and 38.1 mL/100 mL/min, respectively. This case was also assessed as a RECIST-based non-responder for systemic arterial and total perfusions and as true positive. However, this case was evaluated as responder and as false positive based on pulmonary arterial perfusion findings. (d) PET/CT shows high uptake of 2-[fluorine-18]-fluoro-2-deoxy-d-glucose, and SUVmax was evaluated as 4.7. This case was evaluated as a RECIST-responder and assessed as false negative. PR, partial response; MPR, multiplanar reformatted; RECIST, Response Evaluation Criteria in Solid Tumors; CE, contrast-enhanced; SUV, standardized uptake value; PET, positron emission tomography.