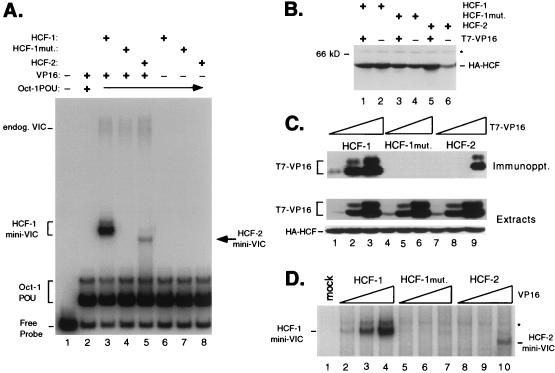
FIG. 6.
The β-propeller domain of HCF-2 is capable of supporting VP16-induced complex formation. (A) HCF polypeptides were coexpressed with VP16ΔC by transfection of 293T cells. Whole-cell extracts were prepared after 40 h and assayed for stabilization of the VP16-induced complex in an electrophoretic mobility shift assay. Lane 1 contains probe alone, and lanes 2 to 5 contain probe with Oct-1 POU domain proteins and the following cell extracts: mock transfection (lane 2), wild-type HCF-1N380 and VP16ΔC (lane 3), HCF-1N380 P134S and VP16ΔC (lane 4), HCF-2N373 and VP16ΔC (lane 5), wild-type HCF-1N380 alone (lane 6), HCF-1N380P134S alone (lane 7), and HCF-2N373 alone (lane 8). The positions of the free probe, Oct-1 POU domain complex, and VP16-induced complex containing native human HCF-1 (endog. VIC) or truncated HCF (HCF-1 or HCF-2 mini-VIC) are indicated. mut., mutant. (B) Immunoblot of extracts used for panel A. Extracts were resolved on an SDS–10% polyacrylamide gel, transferred to nitrocellulose, and immunoblotted with a monoclonal antibody against HA (12CA5). The size of a molecular weight marker is indicated on the left, and a nonspecific cross-reacting protein is indicated with an asterisk. (C) HCF-VP16 coimmunoprecipitation. Direct interaction between HCF-2 and VP16ΔC was examined by coimmunoprecipitation assay. Extracts were prepared from 293T cells transfected with 10 ng, 100 ng, and 1.0 μg of T7-tagged VP16ΔC expression plasmid together with a constant 3 μg of expression plasmids encoding HA-tagged wild-type HCF-1N380 (lanes 1 to 3), P134S HCF-1N380 (lanes 4 to 6), and HCF-2N373 (lanes 7 to 9). Extracts were precipitated with an anti-HA antibody, and VP16 was detected by immunoblotting with the anti-T7 epitope tag antibody. Direct immunoblotting of the extracts (lower two panels) showed that all HA-tagged HCF polypeptides were expressed at equivalent levels and that the expression of T7-tagged VP16 was proportional to amount of input plasmid. (D) Transfected cells extracts used for panel C were assayed for VP16-induced complex formation by electrophoretic mobility shift assay. Labeled TAATGARAT-containing probe and the Oct-1 POU domain were mixed with the following transfected cell extracts: mock transfection (lane 1), HCF-1N380 with 10 ng (lane 2), with 100 ng (lane 3), and with 1.0 μg of VP16ΔC expression plasmid (lane 4), HCF-1N380P134S with 10 ng (lane 5), with 100 ng (lane 6), and with 1.0 μg of VP16ΔC expression plasmid (lane 7), and HCF-2 with 10 ng (lane 8), with 100 ng (lane 9), and with 1.0 μg of VP16ΔC expression plasmid (lane 10). For clarity, only the VP16-induced complexes produced by recombinant HCF (labeled HCF-1 and HCF-2 mini-VIC) are shown. A nonspecific complex is indicated with an asterisk.