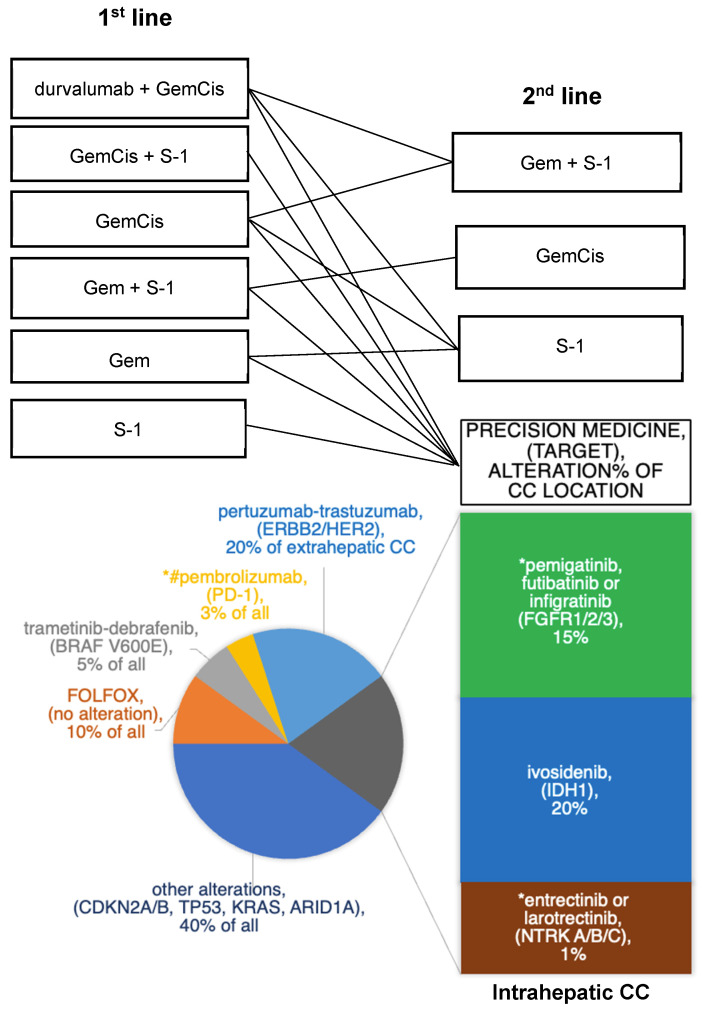
Figure 3.
Current standard therapy for unresectable biliary tract cancer, including intrahepatic cholangiocarcinoma, in Japan and the rates of viable genomic targets. The size of the colored sections of this pie chart was determined approximately for each genomic target. The numerical value % within each section is relative to the genetic alteration by location of cholangiocarcinoma onset, including overlaps. Abbreviations: all, intrahepatic/extrahepatic CC and gallbladder cancer; ARID, AT-rich interaction domain; BRAF, B-serine/threonine kinase; CC, cholangiocarcinoma; CDKN, cyclin-dependent kinase inhibitor; Cis, cisplatin; ERBB/HER, erythroblastic oncogene B/human epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; FOLFOX, 5-fluorouracil/folinic acid-oxaliplatin; Gem, gemcitabine; IDH, isocitrate dehydrogenase; KRAS, Kirsten rat sarcoma; NTRK, neurotrophic tyrosine receptor kinase; PD-1, programmed cell death protein 1; S-1, oral 5-fluorouracil (approved only in Japan); #, microsatellite instability-high; *, approved in Japan.