Abstract
Purpose:
Glaucoma is the second leading cause of blindness worldwide, affecting more than 64 million people aged 40–80. The best way to manage primary open-angle glaucoma (POAG) is by lowering the intraocular pressure (IOP). Netarsudil is a Rho kinase inhibitor, the only class of antiglaucoma medications that reorganizes the extracellular matrix to improve the aqueous outflow through the trabecular pathway.
Methods:
An open-label, real-world, multicentric, observation-based 3-month study was performed for assessing the safety and ocular hypotensive efficacy of netarsudil ophthalmic solution (0.02% w/v) in patients with elevated IOP. Patients were given netarsudil ophthalmic solution (0.02% w/v) as a first-line therapy. Diurnal IOP measurements, best-corrected visual acuity, and adverse event assessments were recorded at each of the five visits (Day-1: screening day and first dosing day; subsequent observations were taken at 2 weeks, 4 weeks, 6 weeks, and 3 months).
Results:
Four hundred and sixty-nine patients from 39 centers throughout India completed the study. The mean IOP at baseline of the affected eyes was 24.84 ± 6.39 mmHg (mean ± standard deviation). After the first dose, the IOP was measured after 2, 4, and 6 weeks, with the final measurement taken at 3 months. The percentage reduction in IOP in glaucoma patients after 3 months of once-daily netarsudil 0.02% w/v solution use was 33.34%. The adverse effects experienced by patients were not severe in the majority of cases. Some adverse effects observed were redness, irritation, itching, and others, but only a small number of patients experienced severe reactions, as reported in a decreasing order: redness > irritation > watering > itching > stinging > blurring.
Conclusion:
We found that netarsudil 0.02% w/v solution monotherapy when used as the first-line treatment in primary open-angle glaucoma and ocular hypertension was both safe and effective.
Keywords: Glaucoma, IOP, netarsudil, ocular hypertension, Rho kinase inhibitors
Glaucoma is a chronic, progressive optic neuropathy characterized by loss of retinal ganglion cells, resulting in characteristic optic nerve head cupping and visual field defects. By 2040, it is anticipated that the global glaucoma burden will increase by more than 110 million.[1] It is the second leading cause of blindness worldwide, affecting more than 64 million people aged 40–80.[2] Furthermore, it is the leading cause of irreversible blindness in India, with at least 12 million people affected and approximately 1.2 million blind. According to the Directorate General of Health Services, the Ministry of Health and Family Welfare of India reports that glaucoma leads to blindness in 5.80% of cases. More than 90% of glaucoma cases in the community remain undiagnosed. In addition, the prevalence of glaucoma increases with age.[3] According to the World Health Organization (WHO), there are several types of glaucoma; however, the two most common are primary open-angle glaucoma (POAG), having a slow and insidious onset, and angle-closure glaucoma (ACG), which is less common and tends to be more acute.[3]
Intraocular pressure (IOP) elevation is a major risk factor for POAG, and in most cases, the only way to slow or stop disease progression is to use pharmaceuticals to reduce IOP. Lowering IOP is the best way to manage POAG. The main contributors to IOP are episcleral venous pressure (EVP), aqueous humor flow rate, and resistance to outflow. IOP is regulated by a fine balance between aqueous humor production and the rate of aqueous outflow through the trabecular meshwork (TM) outflow and the uveoscleral outflow pathway. The majority of the aqueous humor is filtered through the TM pathway. Instead of directly targeting the TM pathway, current therapeutic options primarily increase uveoscleral outflow or decrease aqueous humor production to lower IOP. The TM is abnormal in POAG and ocular hypertension (OHT), but few medications specifically address this issue. Inadequate IOP treatments exist, and older drugs that targeted the TM either had a limited effect or were poorly tolerated.
Netarsudil is a Rho kinase inhibitor, the only class of antiglaucoma medications that reorganizes the extracellular matrix to improve the aqueous outflow through the trabecular pathway. Its multimodal mechanism also includes a decrease in aqueous secretion and EVP. Furthermore, netarsudil is also believed to possess inhibitory action against the norepinephrine transporter (NET). Inhibition of NET prevents the reuptake of norepinephrine at the noradrenergic synapses, which increases the strength and duration of endogenous norepinephrine signaling. As a consequence of this enhanced signaling, norepinephrine-induced vasoconstriction that can reduce blood flow to the ciliary body may subsequently be responsible for a mechanism in which the formation of aqueous humor may be delayed, prolonged, or reduced as well.[4]
Major trials (like ROCKET-1, ROCKET-2) have evaluated the efficacy and safety of netarsudil in patients with open-angle glaucoma (OAG) or OHT. ROCKET-4 compared the efficacy and safety profile of netarsudil once daily versus timilol twice daily.
Using a post-marketing surveillance trial, we evaluated the real-world efficacy of netarsudil ophthalmic solution (0.02% w/v) monotherapy as the first treatment in Indian patients to determine its safety and ocular hypotensive efficacy in patients with elevated IOP.
Methods
Study design
An open-label, real-world, multicentric, observation-based 3-month study for assessing the safety and ocular hypotensive efficacy of netarsudil ophthalmic solution (0.02% w/v) in patients with elevated IOP was performed using Goldmann applanation tonometry (GAT). The 6-month study involved 39 clinical sites in India (1 month enrollment, 3 months study period, and 2 months of data collection as well as analysis). Netarsudil ophthalmic solution (0.02% w/v) was given to patients as a first-line therapy.
Eligibility criteria
Adults (18 years of age or greater) with a diagnosis of POAG or OHT, deemed to be appropriate for starting netarsudil at the treating ophthalmologist’s discretion, were included in the study. At the qualification visit, unmedicated IOP had to be >17 mmHg and <27 mmHg. Each eye had 20/200 vision. The eye with the higher IOP was chosen for the study. If both eyes had the same IOP, the right eye was studied. Both eyes may be studied depending on the ophthalmologist’s individual choice.
Exclusion criteria
Pseudoexfoliation, pigment dispersion, angle closure, or narrow angles were excluded (previous laser peripheral iridotomy). IOP ≥27 mmHg (unmedicated) in both eyes or more than two ocular hypotensive medications within 30 days of screening (two drugs make up fixed-dose combinations). Patients with known hypersensitivity to any formulation component, previous glaucoma intraocular surgery or glaucoma laser procedures in either eye, refractive surgery in either eye, ocular trauma in either eye within 6 months before screening, ocular surgery or nonrefractive laser treatment within 3 months before screening, and recent or current ocular infection or inflammation in either eye were not included.
Current clinically significant blepharitis, conjunctivitis, or a history of herpes simplex or zoster keratitis at screening in either eye, ocular medication in either eye of any kind within 30 days of screening, clinically significant ocular disease in either eye that might interfere with the study, including glaucomatous damage so severe that washout of ocular hypotensive medications for 1 month is not judged safe, and patients with central corneal thickness in either eye greater than 600 μm at screening were excluded. Exclusion criteria included any eye abnormality that prevented reliable applanation tonometry. Patients were excluded if they had clinically systemic abnormalities (as determined by the investigator) in laboratory tests at screening that may impact the study; a clinically significant systemic disease that may interfere with the study; or changes in systemic medication that could affect IOP within 30 days before screening or during the study. The primary efficacy outcome was mean IOP, whereas the secondary outcome was exposure to study medication in days for all treatment groups. The primary endpoint was a change in IOP at the end of 3 months.
Method
Diurnal IOP was measured by GAT in all centers. Also, best-corrected visual acuity and adverse event assessments were recorded at each of the five visits (Day-1: screening day and first dosing day; subsequent observations were taken at 2 weeks, 4 weeks, 6 weeks, and 3 months). From each of the 39 centers, a consolidated set of real-world practice data was collected and statistically analyzed.
Results
Four hundred and sixty-nine patients from 39 centers throughout India participated in the study. The mean IOP at baseline was 24.84 mmHg for the affected eye (mean ± standard deviation). The IOP was measured after 2, 4, and 6 weeks, with the final measurement taken 3 months after the first dose. As shown in Fig. 1 and Table 1, the IOP of the affected eyes was 16.56 mmHg. The percentage reduction in IOP in glaucoma patients after 3 months of once-daily netarsudil 0.02% w/v solution use was 33.34%.
Figure 1.
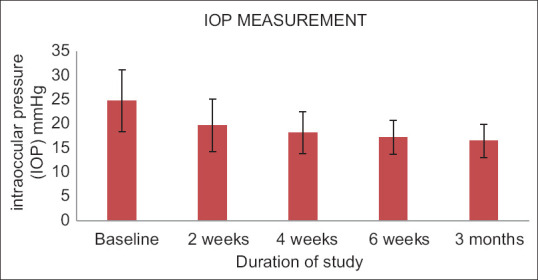
Changes in IOP in mmHg observed in both eyes. IOP = intraocular pressure, LE = left eye, RE = right eye
Table 1.
Measured IOP in mmHg
| Baseline | 2 weeks | 4 weeks | 6 weeks | 3 months | |
|---|---|---|---|---|---|
| IOP of the affected eye | IOP of the affected eye | IOP of the affected eye | IOP of the affected eye | IOP of the affected eye | |
| Mean±standard deviation | 24.84±6.39 | 19.73±5.44 | 18.20±4.34 | 17.31±3.57 | 16.56±3.43 |
n=469 patients at 39 centres; STD DEV=Standard deviation. IOP=intraocular pressure
In the majority of cases, adverse effects experienced by patients were not severe. Patients’ irritation, itching, redness, blurred vision, stinging, and tear production were classified as mild, moderate, severe, or nonexistent [Fig. 2 and Table 2]. Sixty percent of patients had their right eye examined, while 40% of patients had their left eye examined.
Figure 2.
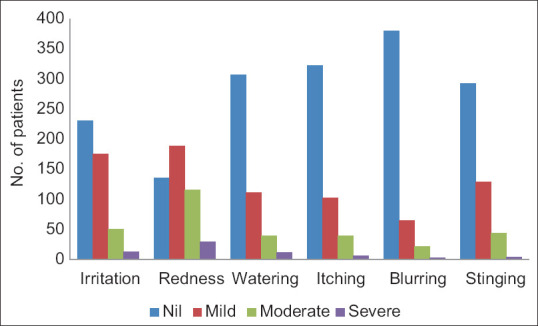
Observed adverse effects
Table 2.
Observed adverse effects
| Nil | Mild | Moderate | Severe | |
|---|---|---|---|---|
| Irritation | 231 | 175 | 50 | 13 |
| Redness | 135 | 189 | 116 | 29 |
| Watering | 307 | 111 | 39 | 12 |
| Itching | 322 | 102 | 39 | 6 |
| Blurring | 380 | 65 | 21 | 3 |
| Stinging | 292 | 129 | 44 | 4 |
n=469 patients at 39 centers
Discussion
The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) approved netarsudil ophthalmic solution (0.02%) for glaucoma in December 2017. This medication lowers IOP in OAG and OHT patients. Netarsudil has no off-label uses. Netarsudil is a once-daily 0.02% ophthalmic solution. IOP is controlled by the balance between aqueous humor production and outflow via the TM and uveoscleral pathways. TM filters most of the aqueous humor.[5]
Lowering the IOP improves the optic disk health not only in glaucoma patients with high IOP (>21 mmHg), but also in those with optic disk changes and normal IOP (i.e., patients with normal-tension glaucoma). Reducing the IOP reduces aqueous humor’s mechanical strain on the eye’s posterior structures, halting glaucomatous optic disk changes. β-adrenergic blockers, α-adrenergic agonists, and carbonic anhydrase inhibitors can reduce the rate of aqueous humor production. Cholinergic drugs (primarily trabecular outflow), α-adrenergic agonists, and prostaglandin (PG) analogs can improve aqueous humor drainage (largely uveoscleral outflow).[6]
Netarsudil reduces fibrotic material deposition in the TM and relaxes the overall tone of contractile cells, enhancing aqueous humor outflow and reducing the IOP.[7] Netarsudil reduces aqueous humor production and improves drainage (trabecular pathway) by inhibiting NET, which lowers the IOP.[8] Netarsudil topical has good corneal penetration. Eye esterases metabolize it to netarsudil-M1, a five-fold more potent active metabolite.[7,8] The IOP-lowering effect of netarsudil and its metabolite peaks 8 h after dosing and lasts 24 h. Once-daily netarsudil lowered the IOP across all baseline pressures. Netarsudil and its active metabolite are highly protein bound (>60%) to plasma proteins. Netarsudil has a 3-h half-life (approximately 175 min).
Consequently, the active duration of IOP reduction justifies a single daily dose of netarsudil 0.02% w/v solution. Similar to previous studies in the American population by Zaman et al.,[9] our findings demonstrate the effect of netarsudil on IOP reduction in OAG and OHT in the Indian population. The safety and efficacy of netarsudil 0.02% w/v solution over 3 months were excellent, and minimal treatment-related adverse effects were observed.[10] Some adverse effects observed were redness, irritation, itching, and others, but only a small number of patients experienced severe reactions, as reported in a decreasing order: redness > irritation > watering > itching > stinging > blurring. In 60% of patients, the right eye was examined, while in 40% of patients, the left eye was examined [Fig. 3]. The pharmacology and dynamic IOP-lowering effect of netarsudil monotherapy as the First-line treatment in Indian patients suggest its utility in the treatment of OAG and OHT.
Figure 3.
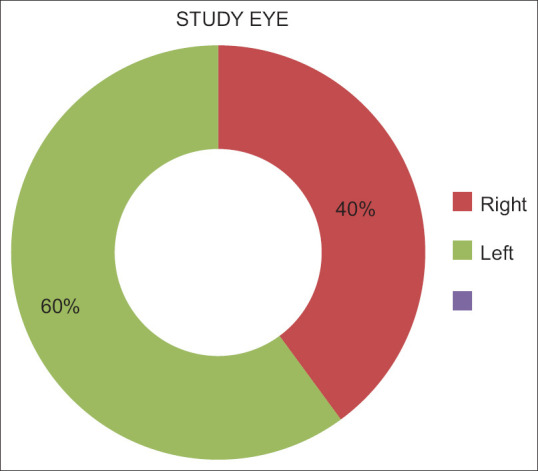
Eyes examined at 39 centers; right = right eye, left = left eye
Adverse effects experienced by patients were not severe in the majority of cases.
Possible retinal side effects were not evaluated in this study because retinal examination before and after administering netarsudil was not included in this study protocols. This should be considered as a limitation of this study.
Conclusion
We found that netarsudil 0.02% w/v solution when used as monotherapy as the first-line treatment in POAG and OHT was both safe and effective.
Financial support and sponsorship
This study was funded by Ajanta Pharmaceuticals Inc.
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Medwiz Healthcare Communication Pvt. Ltd.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors would like to thank all patients who participated in this study.
References
- 1.Khouri AS, Serle JB, Bacharach J, Usner DW, Lewis RA, Braswell P, et al. Once-daily netarsudil versus twice-daily timolol in patients with elevated intraocular pressure: The randomised phase 3 ROCKET-4 study. Am J Ophthalmol. 2019;204:97–104. doi: 10.1016/j.ajo.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. [Lat accessed on 2022 Sep 22]. Available from: https://www.nhp.gov.in/world-glaucoma-week-022_pg#:~:text=Glaucoma%20is%20the%20 second%20most,people%20blind%20from%20the%20disease .
- 4.Kazemi A, McLaren JW, Kopczynski CC, Heah TG, Novack GD, Sit AJ. The effects of netarsudil ophthalmic solution on aqueous humor dynamics in a andomized study in humans. J Ocul Pharmacol Ther. 2018;34:380–6. doi: 10.1089/jop.2017.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anonymous. [Last accessed on 2022 Sep 22]. Available from: https://www.statpearls.com/ArticleLibrary/viewarticle/133789 .
- 6.Gonzalez LE, Boylan PM. Netarsudil for the treatment of open-angle glaucoma and ocular hypertension: A literature review. Ann Pharmacother. 2021;55:1025–36. doi: 10.1177/1060028020971215. [DOI] [PubMed] [Google Scholar]
- 7.Ghanghas RR, Mohan P, Sharma V, et al. Netarsudil: A novel intra-ocular pressure lowering agent. Int J Basic Clin Pharmacol. 2018;7:2268–70. [Google Scholar]
- 8.Sit AJ, Gupta D, Kazemi A, McKee H, Challa P, Liu KC, et al. Netarsudil improves trabecular outflow facility in patients with primary open angle glaucoma or ocular hypertension: A phase 2 study. Am J Ophthalmol. 2021;226:262–9. doi: 10.1016/j.ajo.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Zaman F, Gieser SC, Schwartz GF, Swan C, Williams JM. A multicenter, open-label study of netarsudil for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension in a real-world setting. Curr Med Res Opin. 2021;37:1011–20. doi: 10.1080/03007995.2021.1901222. [DOI] [PubMed] [Google Scholar]
- 10.Singh IP, Fechtner RD, Myers JS, Kim T, Usner DW, McKee H, et al. Pooled efficacy and safety profile of netarsudil ophthalmic solution 0.02% in patients with open-angle glaucoma or ocular hypertension. J Glaucoma. 2020;29:878–84. doi: 10.1097/IJG.0000000000001634. [DOI] [PMC free article] [PubMed] [Google Scholar]


