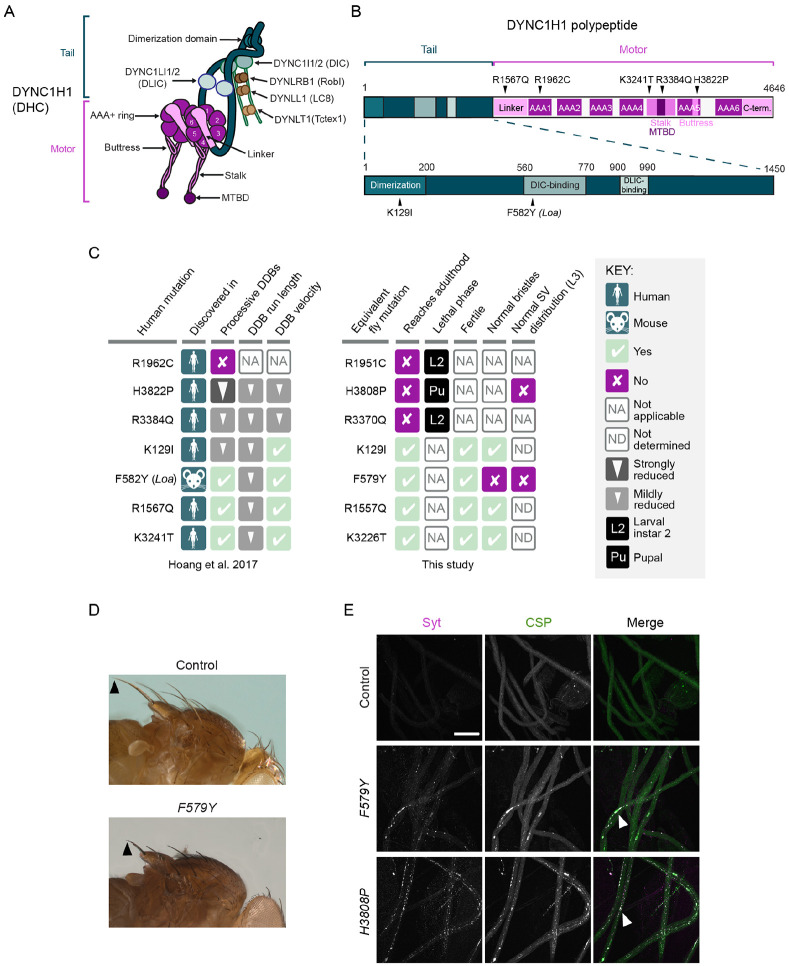
Figure 1. Dynein organization and phenotypic analysis of Dhc disease-associated mutations in Drosophila.
(A) Cartoon of human dynein complex with alternative nomenclature for subunits shown. MTBD, microtubule-binding domain. The C-terminal domain of DYNC1H1 is not visible in this view as it lies on the other face of the AAA+ rings. (B) Positions in the human DYNC1H1 polypeptide of the disease-associated mutations characterized in this study. Subdomains of the DYNC1H1 polypeptide are color-coded as in panel A. The mouse Loa mutation is numbered according to the equivalent residue in human DYNC1H1. Adapted from Hoang et al, 2017. (C) Summary of in vitro and in vivo effects of disease-associated mutations. DDB, dynein-dynactin-BICD2N; SV, synaptic vesicle; L3, larval instar 3. In vitro effects refer to observations when both copies of DYNC1H1 in the dynein complex contain the mutation; in vivo phenotypes refer to the homozygous condition. (D) Images showing short bristles on the notum of homozygous DhcF579Y adult flies compared to controls (yw). Arrowheads point to posterior scutellar macrochaetae as an example. The bristle phenotype of DhcF579Y flies was completely penetrant (>160 flies examined). (E) Confocal images of segmental nerves (taken proximal to the ventral ganglion; anterior to the top; Z-projections) from fixed L3 larvae stained for the synaptic vesicle proteins Synaptotagmin (Syt) and Cysteine-string protein (CSP). Arrowheads show examples of synaptic vesicle accumulations in mutants. Images are representative of 3 – 6 larvae analyzed per genotype. Scale bar: E, 50 μm.