FIG 1.
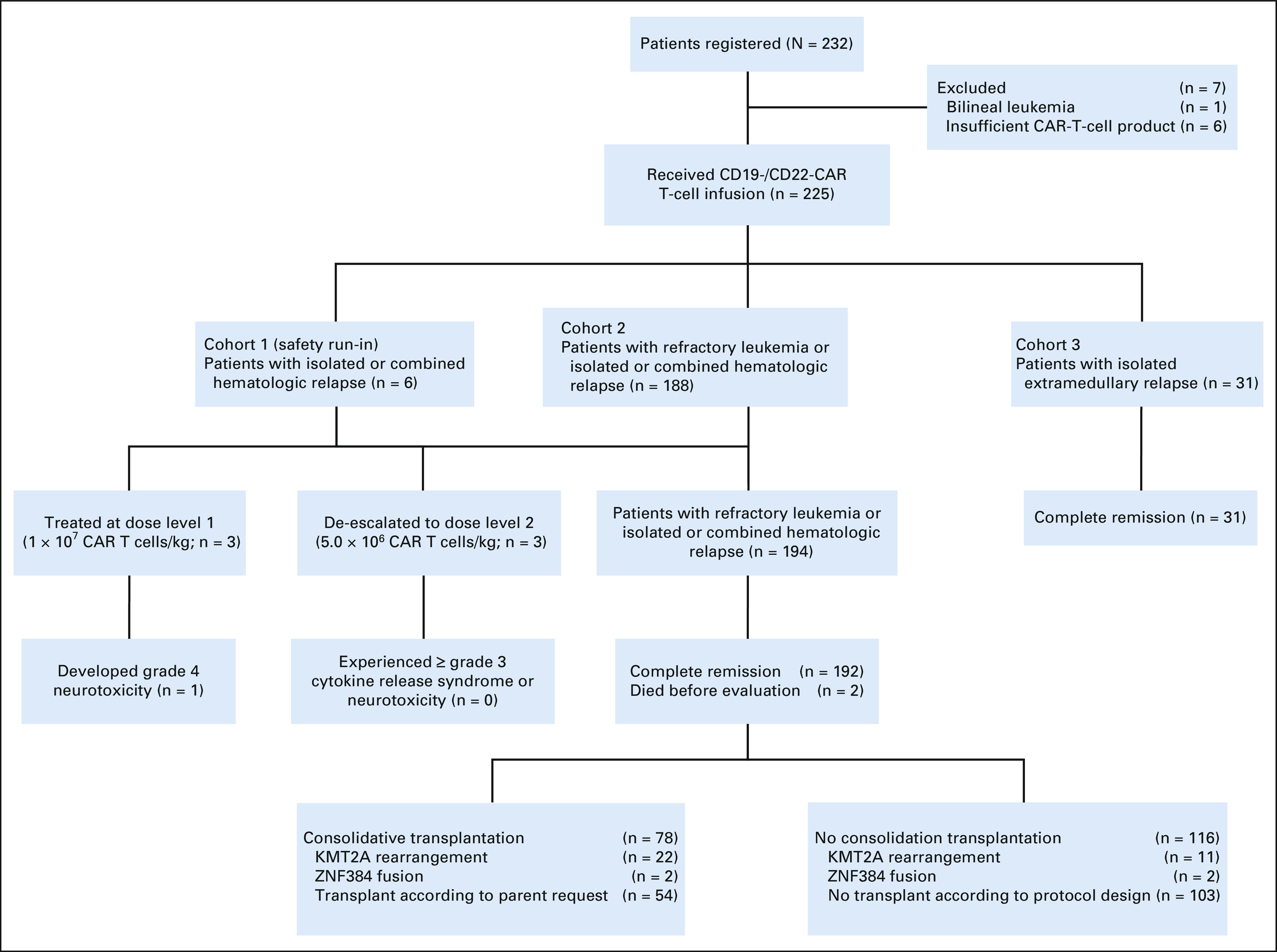
CONSORT diagram. Seven patients were excluded from the study because of the diagnosis of acute bilineal leukemia or insufficient CAR T-cell production (< 1 × 106 CAR T cells/kg). The first six patients with hematologic relapse (cohort 1) were treated in the safety run-in stage. Subsequent 188 patients with refractory leukemia or hematologic relapse (cohort 2) were treated with recommended phase II dose. Among the total 194 patients in cohort 1 and cohort 2, 192 achieved complete remission, of whom 78 received consolidative transplantation. All 31 patients with isolated extramedullary disease (cohort 3) achieved complete remission. CAR, chimeric antigen receptor.
