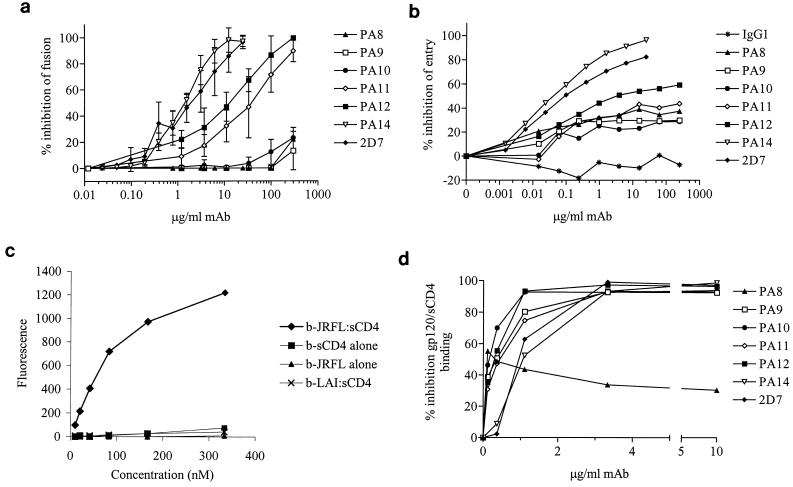
FIG. 4.
Inhibition of CCR5 coreceptor function by anti-CCR5 MAbs. Inhibition of cell-cell fusion by anti-CCR5 MAbs was tested in the RET assay (a). A total of 0 to 250 μg of PA8 to PA12 per ml or 0 to 25 μg of PA14 or 2D7 per ml was added to a mix of HeLa-EnvJR-FL+ and PM1 cells. Results are mean RET values from three independent experiments and are expressed as percent inhibition of fusion = [1 − (% RET in the presence of MAb/% RET in the absence of MAb)] × 100%. Inhibition of HIV-1 entry by anti-CCR5 MAbs was tested in a single-round replication luciferase-based entry assay (b). U87-CD4+ CCR5+ cells were infected with NLLuc+ Env− reporter virus carrying the JR-LF envelope in the presence of 0 to 250 μg of PA8 to PA12 per ml or 0 to 25 μg of PA14 or 2D7 per ml. Luciferase activity (RLU) was measured in cell lysates 72 h postinfection. Results are from a representative experiment and are expressed as percent inhibition of entry = [1 − (RLU in the presence of MAb/RLU in the absence of MAb)] × 100%. Shown is binding of biotinylated (b) gp120, sCD4, and b-gp120-CD4 complexes to L1.2-CCR5+ cells (c). Strong binding is observed when gp120 derived from the R5 virus HIV-1JR-LF is complexed with an equimolar amount of sCD4. No binding is observed in the absence of sCD4 or for gp120 derived from the X4 virus HIV-1LAI. Background binding to CCR5-L1.2 cells has been subtracted from all curves. Inhibition of gp120-sCD4 binding to L1.2-CCR5+ cells was tested in the presence of varying concentrations of each antibody (d). Cells were preincubated in 96-well plates with an anti-CCR5 MAb followed by an incubation with a saturating concentration of biotinylated gp120-sCD4. Finally, binding of PE-labeled streptavidin to cells was measured with a fluorescence plate reader. Results are from a representative experiment and are expressed as percent inhibition of gp120-sCD4 binding = [1 − (MFI in the presence of MAb/MFI in the absence of MAb)] × 100%.