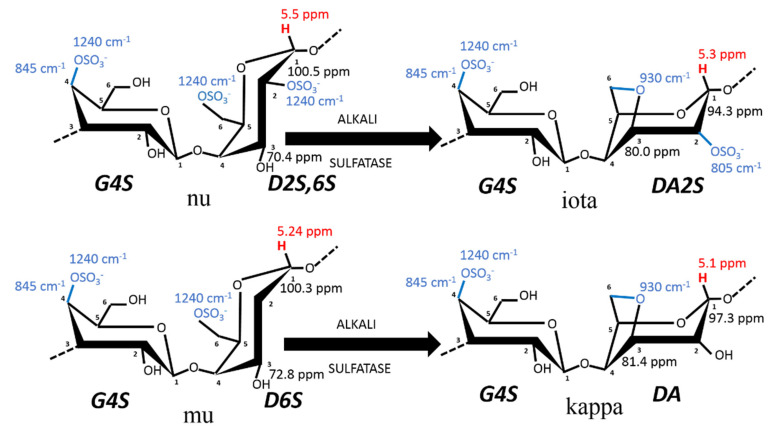
Figure 1.
Chemical structure of carrageenan disaccharides comprising the sulfated polysaccharides K, I, and K2. K2 contains between 20 and 50 mol% of G4S-DA2S, as arbitrarily set by the industrial definition of kappa-2 or weak kappa [9]. The diads given for each carrageenan structure follow the nomenclature introduced by Knudsen et al. [13], where numbers close to carbons indicate their numeration in the galactopyranose. For instance, in iota-carrageenan made of alternating residues of 3-linked β-D-galactopyranose (G) sulfated at the fourth carbon (4S) and 4-linked 3,6-anhydrogalactose-D-galactopyranose (DA) sulfated at the second carbon (2S) is noted as G4S-DA2S, whereas in biological precursors, nu- and mu-carrageenan, the 4-linked α-D-galactopyranose is noted as D, with the same nomenclature for indicating the positions of the sulfates. The transformation from (D) into (DA) can be performed in the industry by alkali treatment (ALKALI) of the seaweed or occurs enzymatically (SULFATASE) in vivo, to turn the biological precursors nu-carrageenan (N) and mu-carrageenan (M) disaccharides into less sulfated I and K, respectively. Note that G4S is common to all of these disaccharides and, thus, characterizes this family of gelling carrageenans. The blue numbers refer to the FTIR absorption bands, which characterize the corresponding chemical bonds highlighted in blue, whereas the red numbers correspond to the 1H-NMR chemical shifts of the corresponding anomeric proton highlighted in red. The numbers close to C1 and C3 of the 4-linked residues correspond to the 13C-NMR chemical shifts of the corresponding carbon.