Abstract
Oral calcium and calcium plus vitamin D supplements are commonly prescribed to several groups of patients, e.g., osteoporosis, fracture, and calcium deficiency. Adequate and steady extracellular calcium levels are essential for neuronal activity, whereas certain forms of calcium supplement (e.g., CaCO3) probably interfere with memory function. However, it was unclear whether a long-term use of ionized calcium (calcium chloride in drinking water ad libitum), vitamin D supplement (oral gavage) or the combination of both affected anxiety and memory, the latter of which was probably dependent on the hippocampal neurogenesis. Here, we aimed to determine the effects of calcium and/or vitamin D supplement on the anxiety- and memory-related behaviors and the expression of doublecortin (DCX), an indirect proxy indicator of hippocampal neurogenesis. Eight-week-old male Wistar rats were divided into 4 groups, i.e., control, calcium chloride-, 400 UI/kg vitamin D3-, and calcium chloride plus vitamin D-treated groups. After 4 weeks of treatment, anxiety-, exploration- and recognition memory-related behaviors were evaluated by elevated pulse-maze (EPM), open field test (OFT), and novel object recognition (NOR), respectively. The hippocampi were investigated for the expression of DCX protein by Western blot analysis. We found that oral calcium supplement increased exploratory behavior as evaluated by OFT and the recognition index in NOR test without any effect on anxiety behavior in EPM. On the other hand, vitamin D supplement was found to reduce anxiety-like behaviors. Significant upregulation of DCX protein expression was observed in the hippocampus of both calcium- and vitamin D-treated rats, suggesting their positive effects on neurogenesis. In conclusion, oral calcium and vitamin D supplements positively affected exploratory, anxiety-like behaviors and/or memory in male rats. Thus, they potentially benefit on mood and memory in osteoporotic patients beyond bone metabolism.
Introduction
Calcium and vitamin D are not only important for bone metabolism but also for certain neural functions, e.g., action potential, neuroplasticity, learning, memory and neuroemotional changes [1–3]. Since inadequate calcium and/or vitamin D intake can increase risk of osteoporosis and fracture, calcium and vitamin D supplements are widely used in osteoporotic patients [4–6]. Dietary calcium supplementation has been reported to increase bone mass and strength in both human [4] and mice [7]. After being absorbed into the body, vitamin D3 is normally converted to hydroxyvitamin D3 [25(OH)D3] and 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], respectively, the latter of which is the active form for enhancement of intestinal calcium absorption (for review, please see [8]). Furthermore, vitamin D supplement is able to modulate energy balance, and the serum 25-hydroxyvitamin D3 [25(OH)D3] levels are linked to body composition [9]. For instance, an increase in 25(OH)D3 level by vitamin D3 supplementation was found to reduce body fat mass [10].
Regarding the effects of calcium and vitamin D on the neuroemotional changes, high serum and cerebrospinal fluid calcium levels were reported to decrease behavioral response and sleep duration [11]. Hypercalcemia due to primary hyperparathyroidism was reportedly associated with mania [12], whereas low serum vitamin D led to anxiety, depression, cognitive impairment, and structural changes in the animal brains [13,14]. Furthermore, an episode of calcium supplementation in drinking water (3 days to 2 weeks), which increased serum and brain calcium levels but decreased brain serotonin turnover, was associated with the enhanced learned helplessness and exploratory behaviors [15,16]. Vitamin D supplement could also alleviate some neurological symptoms in patients with Parkinson’s disease, and improve the depression-like behaviors in ovariectomized rats [17,18]. We thus hypothesized that oral calcium and/or vitamin D supplements with dosages known to keep balancing calcium status may have some beneficial effects on anxiety-like behavior and memory of male rats. It was noteworthy that inappropriate calcium supplementation was able to aggravate cognitive decline or dementia in certain groups of patients, e.g., female elderly with cerebrovascular disease [19].
The hippocampus is crucial for both anxiety response and memory in humans and rodents [20–22], and the hippocampal neurogenesis is often induced by factors that modulate learning and memory in rats. Moreover, a link between vitamin D and hippocampal neurogenesis via calcium signaling has been reported [23–25]. An increase in hippocampal neurogenesis was also found to alleviate anxiety- and depression-like behaviors in stressed mice [26]. Since oral calcium and/or vitamin D supplements may positively affect anxiety response and memory, they were further postulated to modulate the expression of doublecortin (DCX)—a neural system-specific microtubule-associated protein commonly used as an indirect biomarker of hippocampal neurogenesis and neuronal migration. The direct evidence that confirms the positive effects of extracellular calcium on neurogenesis is scant, but there are several purports suggesting that neurogenesis requires optimal levels of both extracellular and intracellular calcium [24,27].
Therefore, the present study aimed to determine the effects of oral calcium supplement (calcium chloride dissolved in drinking water) with or without vitamin D supplement (dissolved in sterile soybean oil; given by gavage) on neurobehavioral changes (e.g., anxiety-like behaviors and object recognition) and the hippocampal DCX protein expression in male rats. The rats were divided into 4 groups accordingly, i.e., control (distilled water), calcium, vitamin D, and calcium + vitamin D (Fig 1A). We only used male rats in the present study in order to avoid variations and interferences owing to ovarian hormones, particularly estrogen-induced upregulation of calcium transporter expression (e.g., TRPV6) in the small intestine [28]. Since calcium/vitamin D supplement is often prescribed for aging patients with osteoporosis, our findings could be potentially translated for further optimization of calcium/vitamin D regimens to best benefit bone metabolism as well as mood and memory.
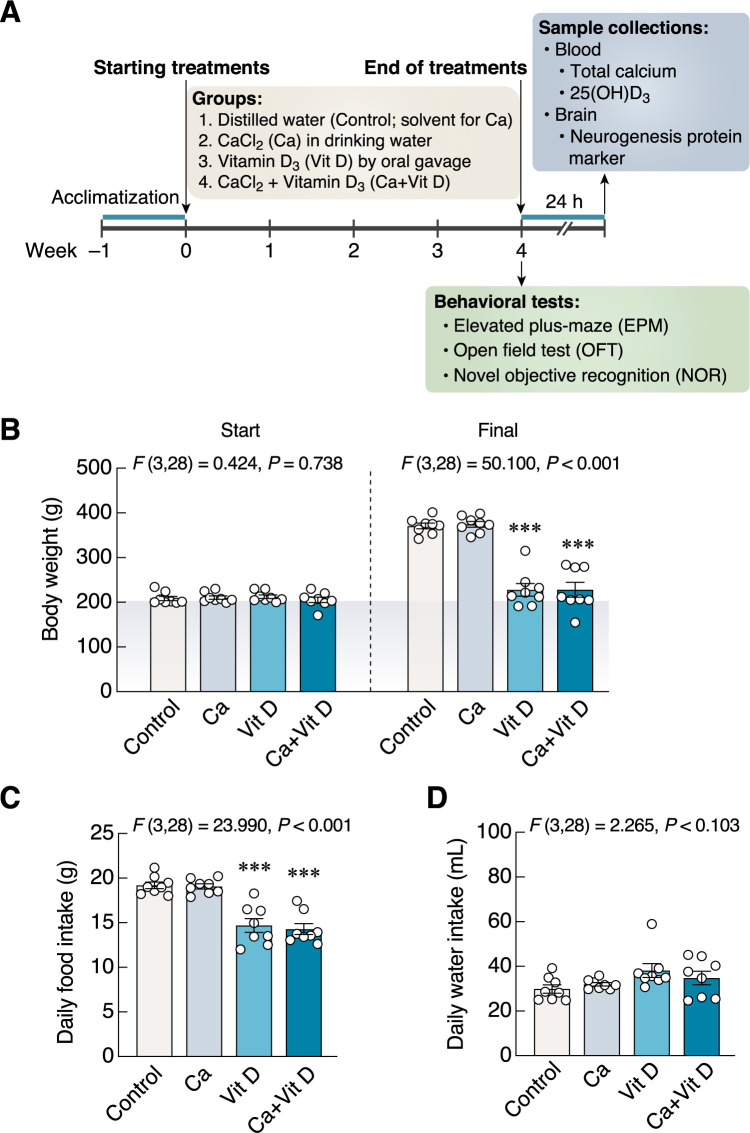
Fig 1. Experimental timeline, changes in body weight, daily food and water intakes.
(A) Time course experiments. After 1-week acclimatization, all rats were assigned into 4 groups: control, calcium, vitamin D, and combined calcium and vitamin D supplements. Rats were treated with drinking water containing calcium chloride ad libitum and/or 400 UI/kg vitamin D3 and/or vehicle (soybean oil) orally via gavage tube for 4 weeks. At the end of treatment period, each rat was individually evaluated for anxiety levels, locomotor activity, and recognition memory by using elevated plus-maze (EPM), open field test (OFT), and novel object recognition (NOR), respectively. After behavioral tests (i.e., 24 h after the last supplementations), all rats were euthanized with isoflurane. (B–D) Changes in body weight, and food and water consumption in rats supplemented with calcium and/or vitamin D3 for 4 weeks. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control. Ca, calcium supplement; Vit D, vitamin D supplement.
Materials and methods
Animals
Eight-week-old male Wistar rats weighing 180–200 g were obtained from Nomura Siam International Co., Ltd., Bangkok, Thailand. The rats were housed in a group of 2–3 rats/cage and acclimatized in controlled room conditions at temperature of 22 ± 1°C, humidity of 50 ± 5%, light intensity of 325 lux, and 12 h light/dark cycle. All rats were fed commercial rat chow (CP Co., Ltd., Bangkok, Thailand) and distilled water ad libitum. This study has been approved and carried out according to the Institutional Care and Use Committee of Thammasat University, Pathumthani, Thailand (Protocol number: 10/2561). Although certain bone diseases, e.g., osteoporosis, often affect females, we avoided using female rats in the present study because cyclical ovarian hormone release may interfere with anxiety-like behaviors and memory [29,30].
Experimental design
After 1 week of acclimatization (Fig 1A), thirty-two rats were randomly divided into 4 groups of 8 rats each. Group 1 (Control) was given distilled water (solvent for calcium) ad libitum and sterile soybean oil (solvent for vitamin D3). Group 2 (Ca) was given calcium chloride in distilled water ad libitum plus soybean oil. Group 3 (Vit D) was given distilled water ad libitum and vitamin D3 dissolved in soybean oil. Group 4 (Ca + Vit D) was given both calcium chloride ad libitum plus vitamin D3 dissolved in soybean oil. In other words, the animals were fed distilled water (control) or calcium-enriched water in drinking bottles ad libitum, and were orally administered vitamin D3 or soybean oil via gavage tube. The last doses of supplements consisting of calcium and/or vitamin D3 were administered 24 h prior to conducting the last behavioral test (i.e., all behavioral tests were performed individually within 24 h after the last supplementation). Twenty-four hours after the end of supplementation, blood samples were collected by cardiac puncture for biochemical analyses [total calcium and 25(OH)D3 levels]. The timing of the individual behavioral tests was between 9:00 and 12:00 a.m., while the timing of the treatment was between 9:00 and 10:00 a.m.
The rats were finally euthanized with isoflurane anesthesia. After removal of whole brain, the hippocampus was collected for measurement of neurogenesis protein levels. DCX as a microtubule-associated phosphoprotein has been used as an indirect biomarker of newly born neurons and dendritic growth in adult dentate gyrus [31,32]. Regarding the DCX protein expression study, we carefully dissected the dorsal hippocampus, which contained the dentate gyrus, according to the modified methods of Vnek and Rothblat, and Heffner et al. [33,34]. Nevertheless, the brain samples may still contain some other hippocampal cells and fibers.
Calcium/Vitamin D supplements
Provision of calcium and vitamin D3 was conducted for 4 weeks (Fig 1A). Rats were administered ad libitum with 15 mM calcium chloride solution (catalog no. 21114, Sigma Chemical Co., St. Louis, MO, USA.) and 12 mM monosaccharides (i.e., glucose; catalog no. 1009378, Ajax Finechem Pty Limited, New South Wales, Australia and galactose; catalog no. 0802339, Asia Pacific Specialty Chemicals Ltd., New South Wales, Australia) and 46.5 mM sodium chloride (catalog no. S5068-1-1000, Quality Reagent Chemical (QrëcTM, New Zealand) according to the formula of Suntornsaratoon et al. (2015) [35]. Distilled water (solvent) or calcium chloride solution was added in drinking bottles for daily treatment.
Vitamin D3 (catalog no. C9756, Sigma Chemical) was dissolved in sterile soybean oil (Thanakorn Vegetable Oil Products Co., Ltd., Thailand) and given orally at 400 IU/kg body weight of rats (soybean oil volume of 0.3 mL), as previously reported by Vieth (2004) and Detregiachi et al. (2016) [36,37].
Body weight, food intake and water intake measurements
Rats were daily weighed in grams. The starting and final body weights of rats were recorded, and changes in daily weight gain were calculated. Food and water intakes were calculated from weight/volume before and after 24 h in the metabolic cage (model 3700M071-01CS; Tecniplast, Varese, Italy).
Elevated plus-maze test (EPM)
The maze was a black plastic open-topped box and elevated 50 cm from the floor. The EPM was composed of two open arms (50 × 10 cm) aligned at a right angle to the perpendicular closed arms (50 × 10 × 40 cm). Each rat was gently placed into the central square of the maze, then allowed to explore the EPM for 5 min. The time spent and number of entries into the open arms were recorded by an infrared camera. A lower value of anxiety index indicated anxiolysis [38–40]. Anxiety index was calculated according to the equation:
Anxiety index = [1–[(open arm time/total time) + (open arm entries/total entries)]]/2
Open field test (OFT)
The open field apparatus was a black plastic box (76 cm long × 57 cm wide × 35 cm high) with grid floor divided into inner and outer zones. Each rat was gently placed in the corner squares and given 5 min to explore. Time spent in each zone of arena, total line crosses, rearing, and grooming were recorded by infrared video camera. Changes in number of the total line crosses and rearing represented exploration and locomotor activity [41,42].
Novel object recognition (NOR)
NOR was performed in a black plastic box (63 cm long × 63 cm wide × 45 cm high) with a video camera suspended above in 360 lux room light. The procedure was performed according to Lapmanee et al., 2017 [42]. After a training session with familiar objects (ceramic bottles), the objects were changed to glass paperweight and ceramic peppermills. Object-exploring behaviors that ran for 3 min consisted of sniffing, licking and touching. A decrease in percent recognition index indicated cognitive impairment, which was calculated according to the equation:
Serum total calcium and 25(OH)D3 analyses
Blood samples (5 mL/animal) were obtained from rats anesthetized with 5% isoflurane (Minrad Inc., New York, USA). Whole blood was placed at room temperature for 30 minutes. After centrifugation (3,000 rpm, 10 min, 4°C), serum samples were collected and kept at –80°C until measurement of total calcium levels by an automated biochemical analyzer (Dimension RxL, Dade Behring Co Ltd., Minnesota, USA) and 25(OH)D3 levels by immunoassay (catalog no. KAP1971; DIAsource ImmunoAssay, Louvain-La-Neuve, Belgium), respectively.
Western blot analysis
Whole brain was removed from the skull and immediately frozen in liquid nitrogen for analysis of protein expression. The hippocampus was dissected following the methods of Heffner et al. (1980) [33]. The hippocampus tissue was mixed in lysis buffer with protease and phosphatase inhibitor cocktails (Sigma). Total protein concentration was determined by using BCA Protein Assay kit (Thermo Scientific Inc., Waltham, MA, USA). Protein samples (50 μg) were added into each well of 10% SDS-PAGE and transferred onto PVDF membranes, methanol soaked (Amersham Biosciences, New Jersey, USA). Thereafter, the membranes were incubated with 1:1,000 rabbit polyclonal anti-doublecortin antibody (catalog no. AB18723; Abcam, Cambridge, UK), or 1:2,000 rabbit polyclonal anti-β-actin antibody (catalog no; AB8227, Abcam) overnight at 4°C. After the washing step, membranes were incubated with 1:2,000 goat anti-rabbit IgG H&L (HRP) secondary antibody (catalog no. AB205718; Abcam) at room temperature for 2 h. Protein bands were visualized by using an enhanced chemiluminescence western blotting detection system (Luminata Crescendo Western HRP substrate; Merck‐Millipore, Darmstadt, Germany) on the X-ray film (Amersham Biosciences). Densitometry was determined by using ImageJ (National Institutes of Health, Bethesda, Maryland, USA). The DCX protein expression level relative to β-actin in the control group was normalized to 1.
Immunofluorescent analysis of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
The TUNEL-positive cells represent apoptotic cells. Therefore, in order to assess apoptosis of cells in the dentate gyrus, TUNEL histochemistry was performed according to the manufacturer’s instruction using CF® dye TUNEL assay apoptosis detection kit (catalog number CF488A; Biotium, CA, USA). Briefly, paraffin brain sections (5 μm in thickness) were deparaffinized and treated with DNaseI before TUNEL staining was set up as positive control. The sections were then mounted with mounting media containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, CA, USA) to stain nuclei, and examined by using Olympus BX51 microscope with fluorescence illuminator (×40 magnification; Olympus, Tokyo, Japan). All positive cells were quantified by counting all cells with fluorescent signals in each histological sample using ImageJ (i.e., counting all cells and nuclei in a section). The percentage of apoptotic cells was calculated from the number of TUNEL-positive cells (green color) and the total number of DAPI-stained nuclei (blue color), as follows.
Statistical analyses
The data are expressed as means ± standard error of mean (SEM). The significance of the differences between groups was analyzed using one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison test. The F-values, degree of freedom (df), and P-values were also presented. The level of significance was P < 0.05. Two-way ANOVA analysis was performed to determine the effects of two factors, i.e., calcium and vitamin D. All statistical tests and graphs were analyzed and plotted by using GraphPad Prism 7 (GraphPad Software Inc., San Diego, CA, USA).
Results
Calcium alone increased urinary calcium levels, while vitamin D3 decreased food intake and increased serum 25(OH)D3 levels
Baseline body weights were similar among the four experimental groups (F(3,28) = 0.424, P = 0.738) (Fig 1B). After 4 weeks of calcium and/or vitamin D supplementation, the final body weights of rats in the vitamin D and combined calcium and vitamin D groups significantly decreased (F(3,28) = 50.100, P < 0.001) (Fig 1B). Consistent with the final body weight, a decrease in daily food intake was observed in the vitamin D group as compared with the control group (F(3,28) = 23.990, P < 0.001) (Fig 1C). Despite showing no statistical significance, the vitamin D group had a tendency to increase daily water intake (F(3,28) = 2.265, P = 0.103) (Fig 1D). We did not observe any overt adverse reactions, e.g., fatigue, abnormal movement, immobility, infectious disease, bleeding, diarrhea, etc.
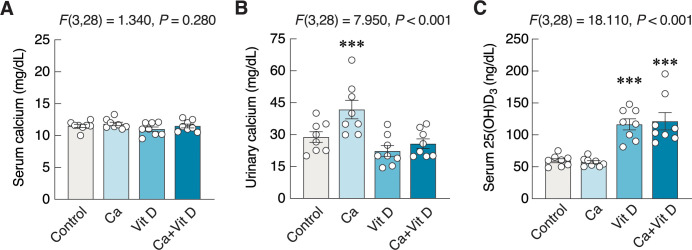
Regarding analyses of urine and serum samples, there was no change in the total calcium level (F(3,28) = 1.340, P = 0.280) (Fig 2A), whereas a significant increase in urinary calcium level was observed in the calcium-treated group (F(3,28) = 7.950, P < 0.001) (Fig 2B). As expected, the two groups of rats that received vitamin D exhibited increases in serum 25(OH)D3 levels as compared to the control group (F(3,28) = 18.110, P < 0.001) (Fig 2C). However, there was no interaction among all parameters in the present study as analyzed by two-way ANOVA. All raw data are also provided as supplementary information.
Fig 2. Changes in calcium and 25-hydroxyvitamin D3 [25(OH)D3] levels.
(A) The serum calcium, (B) urinary calcium, and (C) serum 25(OH)D3 in rats supplemented with calcium and/or vitamin D3 for 4 weeks. ***P < 0.001 vs. control. Ca, calcium supplement; Vit D, vitamin D supplement.
Vitamin D3 alone exhibited anxiolytic-like action
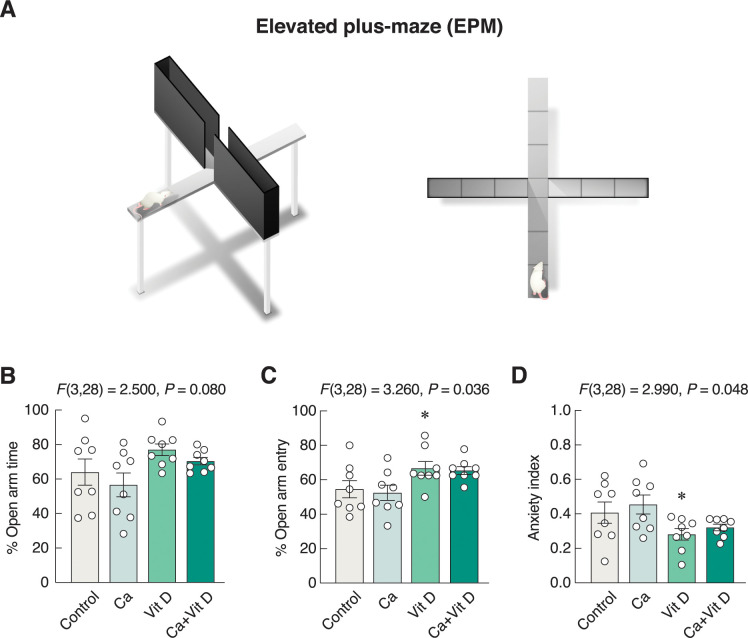
Anxiety-like behavior was determined by EPM as depicted in Fig 3A. The 4-week vitamin D3 supplement alone tended to increase time spent in the open arms (F(3,28) = 2.500, P = 0.080) (Fig 3B) and significantly increased open arm entry (F(3,28) = 3.260, P = 0.036) (Fig 3C). Thus, as shown in Fig 3D, the vitamin D-supplemented group exhibited a lower anxiety index than the control group (F(3,28) = 2.990, P = 0.048), suggesting that it had an anxiolytic-like action.
Fig 3. Changes in anxiety-related behaviors.
(A) The anxiety-like behaviors were evaluated by EPM. (B) The percent open arm time, (C) the percent open arm entry, and (D) the anxiety index in rats supplemented with calcium and/or vitamin D3 for 4 weeks. *P < 0.05 vs. control. Ca, calcium supplement; Vit D, vitamin D supplement.
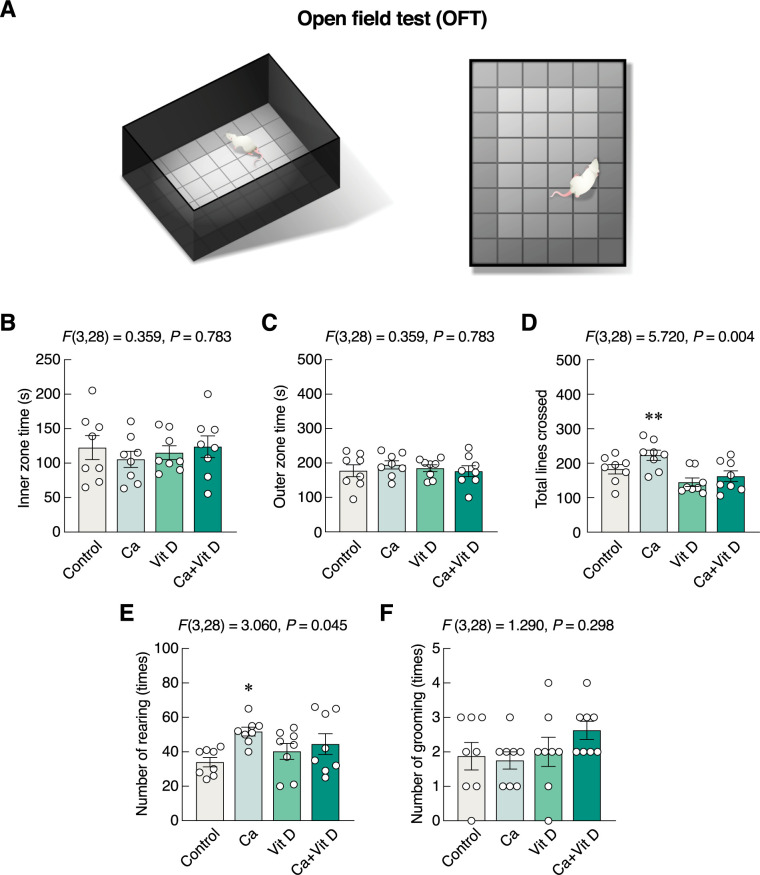
In OFT (Fig 4A), the numbers of line crossing and rearing were used as proxy indicators of locomotor activity. Although none of the supplementations altered time spent in the inner zone (F(3,28) = 0.359, P = 0.783) (Fig 4B), time spent in the outer zone (F(3,28) = 0.359, P = 0.783) (Fig 4C), or number of grooming (F(3,28) = 1.290, P = 0.293) (Fig 4F), calcium-supplemented group showed significant increases in the number of total lines crossed (F(3,28) = 5.720, P = 0.004) (Fig 4D) and number of rearing (Fig 4E), suggesting that calcium supplement alone was able to enhance locomotor activity.
Fig 4. Changes in exploration and locomotor activity.
(A) The behavioral profiles were evaluated by OFT. (B) The inner zone time, (C) outer zone time, (D) number of total line crossed, (E) number of rearing, and (F) number of grooming in rats supplemented with calcium and/or vitamin D3 for 4 weeks. *P < 0.05, **P < 0.01 vs. control. Ca, calcium supplement; Vit D, vitamin D supplement.
Calcium alone improved object recognition memory
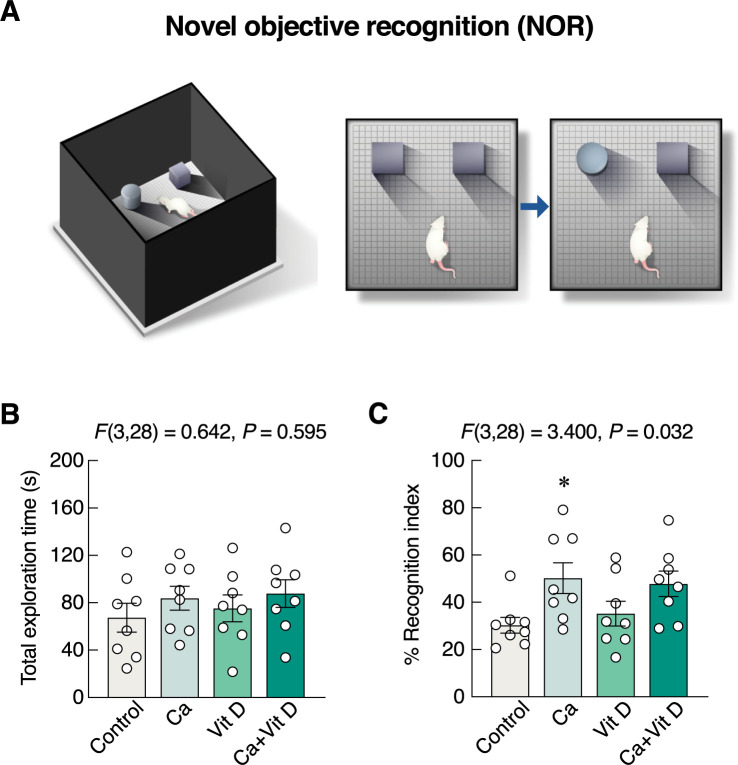
NOR test was used to evaluate the differences between the novel and familiar object exploring times (Fig 5A). There were no significant differences in the total exploration time among the experimental groups (F(3,28) = 0.642, P = 0.595) (Fig 5B). However, the percent recognition index for the novel object in the calcium-supplemented group was higher than that in control group (F(3,28) = 3.400, P = 0.032) (Fig 5C). The aforementioned results suggested that calcium alone, but not vitamin D3, was capable of improving object recognition memory.
Fig 5. Changes in recognition memory.
(A) The behavioral profiles were evaluated by NOR. (B) The total exploration time, and (C) percent recognition index in rats supplemented with calcium and/or vitamin D3 for 4 weeks. *P < 0.05 vs. control. Ca, calcium supplement; Vit D, vitamin D supplement.
Calcium and/or vitamin D3 upregulated hippocampal DCX protein expression
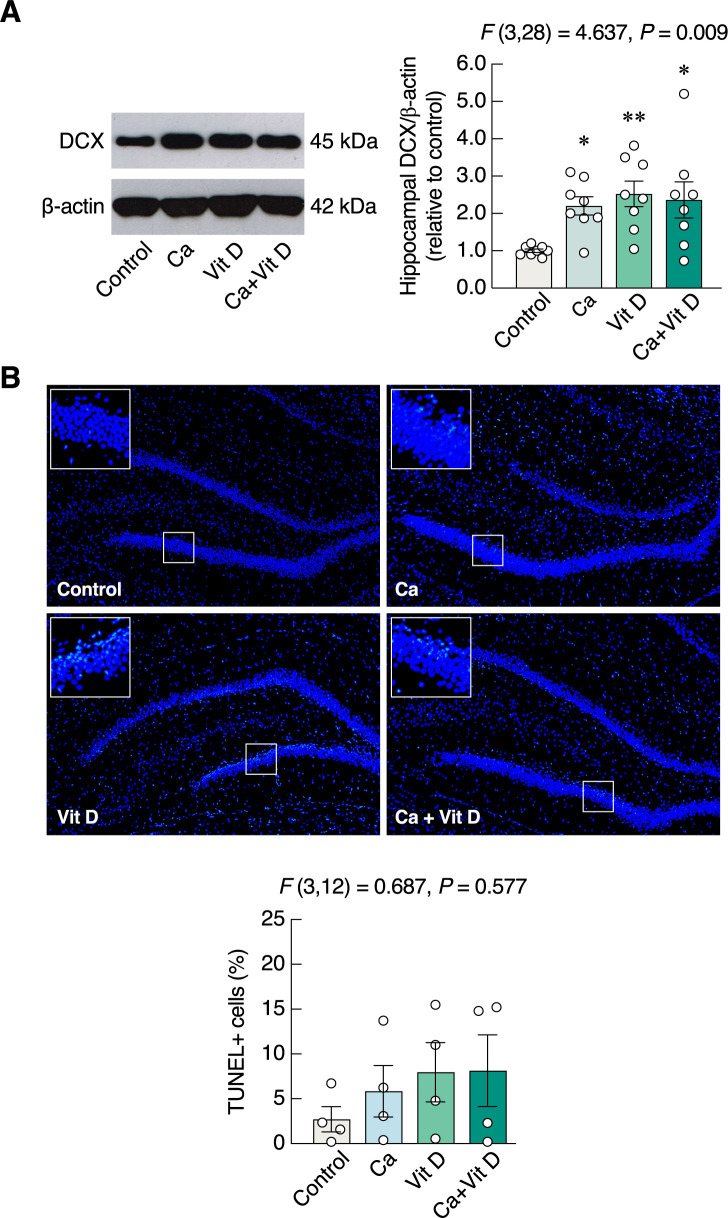
Since hippocampus plays a pivotal role in object recognition memory based on memory formation, the calcium/vitamin D supplements probably helped modulate the hippocampal neurogenesis. Hence, the proxy biomarker of adult neurogenesis—i.e., DCX protein—was determined by Western blotting analysis. We found that the hippocampal DCX protein levels of calcium-supplemented as well as vitamin D-supplemented groups were significantly upregulated (F(3,28) = 4.637, P = 0.009) (Fig 6A), as compared to the control group. DCX protein expression was also upregulated the calcium plus vitamin D group (Fig 6A). Meanwhile, calcium and/or vitamin D did not induce cell apoptosis in dentate gyrus as determined by TUNEL assay (F(3, 12) = 0.687, P = 0.577) (Fig 6B).
Fig 6. Expression of hippocampal doublecortin (DCX) and cell apoptosis in dentate gyrus.
(A) Representative and relative protein expression levels of hippocampal doublecortin (DCX) and β-actin in rats supplemented with calcium and/or vitamin D3 for 4 weeks. (B) Representative fluorescent images (10×) of TUNEL-positive (green color) and nuclei counterstained with DAPI (blue color), and percentage of TUNEL-positive cells relative to total number of nuclei in dentate gyrus. *P < 0.05, **P < 0.01 vs. control. Ca, calcium supplement; Vit D, vitamin D supplement.
Discussion
Our study has provided evidence that showed the positive effects of calcium and/or vitamin D3 on anxiety-like behaviors and object recognition memory. Calcium is one of the nutritional supplements recommended for postmenopausal women to alleviate bone loss and osteoporosis [4,5]. Under normal conditions, since serum calcium levels are tightly regulated and maintained within a narrow physiological range, it was not surprising to observe no change in serum calcium levels among the four groups in the present study. Although acute calcium administration and some calcium formulae might slightly increase serum calcium levels [43], an increase in serum calcium is often transient due to the fact that hypercalcemia is able to induce urinary calcium excretion as seen in Fig 2B. Thus, after 4-week calcium supplementation, the serum calcium levels were apparently normal. Nevertheless, some previous investigations have suggested that calcium intake within normal ranges did not induce hypercalciuria [44], but calcium intake in the present calcium-supplemented group was probably in a higher range, which might suppress parathyroid hormone response, thereby resulting in a reduction in renal calcium reabsorption and enhancing renal calcium excretion [45].
Regarding the behavioral responses, calcium supplementation was found to increase the exploratory behaviors and object recognition memory (NOR test) together with an increase in hippocampal DCX protein expression and no change in apoptosis in dentate gyrus. Our finding partially agreed with previous investigations showing that calcium supplement alone in drinking water was able to increase brain calcium levels (whole brain), which could contribute to high exploration activities [43] and central serotonin levels in rats [16]. The increased calcium levels in cerebral cortex, hippocampus, forebrain and brainstem could facilitate neurogenesis—a process known to increase memory capacity. The enhanced adult neurogenesis in dentate gyrus probably occurred via calcium influx through T-type calcium channels and calcium/calmodulin-dependent protein kinase II modulation [46,47]. Moreover, since there were crosstalks between parathyroid hormone-related protein (PTHrP)/brain calcium metabolism and adrenergic/serotonergic pathways, the underlying mechanisms of neurogenesis were probably related to norepinephrine and serotonin modulation that could increase the excitatory drive to the hippocampal circuit for neurogenesis [15,48–50]. Serotonin directly activated 5-HT3 receptors and also affected the function of PTHrP, which was capable of enhancing brain c-Fos expression and inducing a neuroprotective feedback loop through the L-type calcium channels [51,52]. Xu et al. also showed that a 5-HT3 receptor agonist (phenylbiguanide) was able to upregulate c-Fos expression in cortex and hippocampus [53]. Meanwhile, the binding of PTHrP to PTH/PTHrP receptor was capable of mediating neuroprotection against calcium-induced excitotoxicity [51,52]. Taken together, the present prolonged calcium supplementation might alter brain monoamine levels, thereby enhancing recognition memory and emotionality in rats, consistent with some previous human studies that showed a reduction in dementia risk by dietary calcium supplementation [54].
Apart from calcium supplementation, vitamin D3 is also crucial for the maintenance of bone structure, enhancement of intestinal calcium absorption [8] and brain functions [5,25]. Herein, there were significant increases in the levels of serum 25(OH)D3 in the vitamin D3-supplemented groups (Fig 2C). Under normal conditions, dietary vitamin D3 is transformed into 25(OH)D3 by CYP27A1 and CYP2R1 in several cells, particularly hepatocytes. However, during calcium imbalance with hypocalcemia, a conversion of 25(OH)D3 to 1,25(OH)2D3—the active form vitamin D3—is markedly enhanced by 1α-hydroxylase (CYP27B1) in the renal tubular cells. Although serum 1,25(OH)2D3 level was not determined in the present study, the absence of changes in serum and urinary calcium in the vitamin D-supplemented groups suggested that calcium homeostasis has already been kept in balance, and thus the levels of 1,25(OH)2D3 were probably unaltered [55].
The elevated 25(OH)D3 levels during vitamin D3 supplementation could lead to body fat mass reduction [10,37]. It was reported that vitamin D intake repressed fatty acid synthase activity in adipose tissue [56,57]. In addition, vitamin D was able to enhance lipid oxidation and energy consumption via agouti-related protein/neuropeptide Y [58,59]; therefore, there was a significant reduction in body weight in the groups receiving vitamin D3, but not calcium alone. Indeed, vitamin D3 is able to reduce body weight by a number of mechanisms. For example, it could modulate pancreatic insulin secretion and insulin sensitivity, thereby reducing food intake, white adipose tissue weight and body weight [59–61]. However, a decrease in body weight did not affect locomotor activity in the vitamin D3-supplemented rats, indicating that the present dosage of vitamin D3 supplement was not toxic. Evaluation of altered locomotor activities can be used to indicate toxicity, as reviewed by Gauvin et al. (2019) [62].
Furthermore, vitamin D3 supplement probably produced anxiolytic action, at least in part, by upregulating DCX protein expression, but had no effect on learning and memory. Normally, circulating 25(OH)D3 is capable of passing across the blood-brain barrier and can be converted into 1,25(OH)2D3 in glial cells and neurons [63], suggesting that changes in serum 25(OH)D3 levels eventually modulate neural circuits and brain functions. It was previously reported that the increased levels of serum 25(OH)D3 were correlated with a reduced risk of depression and anxiety disorder [64,65]. The positive findings of vitamin D and a reduction in anxiety were obtained from studies using various behavioral tests, e.g., OFT, the light-dark box, and the elevated plus-maze in the ovariectomized rats [66]. At the cellular level, vitamin D protected and enhanced growth of neuronal cells by inducing production of growth factors, e.g., nerve growth factor, glial cell line-derived neurotrophic factor, and neurotrophin 3 [67,68]. Moreover, it could also modulate biosynthesis of certain neurotransmitters—such as serotonin through tryptophan hydroxylase 2—and neurotrophic factors, thereby modulating mood and anxiety-like behaviors [69]. A number of previous investigations also reported the improvement of mood status, antioxidant and anti-inflammatory responses in vitamin D-treated type 2 diabetic patients [66,70]. Consistent with the behavioral studies, changes in the expression levels of essential molecules for learning and memory as well as neuronal proliferation, differentiation, survival and growth of hippocampal neurons—e.g., nerve growth factor and CREB—were detected [71,72]. In addition, the upregulation of neuronal biomarker of hippocampal neurogenesis could contribute to an alleviation of anxiety- and depression-like behaviors [26]. These data have supported our hypothesis that vitamin D3 supplementation exerted an anxiolytic-like action. Although its exact underlying mechanisms are unclear, hippocampal neurogenesis as indicated by DCX expression probably contributed to the process.
Nevertheless, the exact explanation regarding the absence of change in percent recognition index (Fig 5C) in Ca+Vit D is unclear. It was evident that calcium supplement alone was able to induce some positive effects on brain, e.g., increased exploration activities [15,43]. Although vitamin D often helps enhance the intestinal calcium absorption to increase body calcium, it also triggers a number of counterbalancing mechanisms, e.g., upregulation of fibroblast growth factor (FGF)-23 production, to prevent excessive calcium uptake (for review, please see Wongdee et al. 2021 [8]). In other words, after several weeks of Ca+Vit D treatment, the action of calcium supplement was gradually diminished by the vitamin D-induced negative feedback regulation. However, as shown in Fig 6A, calcium and/or vitamin D plausibly promoted adult neurogenesis, which was sufficient to reduce anxiety-related behaviors and modulate locomotor activities in rats. Specifically, an increase in adult neurogenesis was able to alleviate anxiety and depression-related behaviors through hypothalamic-pituitary-adrenal axis in stressed or depressed rodents [26]. In addition, neurogenesis may also induce exploration behavior [73].
Regarding neuronal apoptosis in dentate gyrus, our TUNEL study indicated that supplementation of calcium chloride in drinking water was not toxic since it did not induce apoptosis of hippocampal neurons. Although certain regimens of calcium supplement, e.g., diet supplemented with 1.0% calcium carbonate (4 g/day; 8-week supplementation) vs. 0.2% calcium carbonate (normal diet), were reported to induce memory impairment in ICR mice as determined by Y-maze test and NOR [74], the present calcium chloride in drinking water ad libitum showed an increase in percent recognition index in rats (Fig 5C), suggesting that the outcome of calcium supplement was probably dependent on calcium compounds (calcium carbonate vs. calcium chloride), duration of treatment (4 vs. 8 weeks), animal species (mouse vs. rat) and/or mode of supplementation (calcium in diet vs. drinking water). Excessive calcium supplementation—particularly with hypercalcemia—is detrimental to neuronal activities. In contrast to the aforementioned beneficial effects of calcium and vitamin D supplements, calcium carbonate was reported to induce memory impairment by decreasing CREB expression, while excess vitamin D level also produced toxicity [74,75]. Moreover, an inappropriately high calcium and/or vitamin D intake can lead to kidney stone formation [76,77]. Therefore, a proper regimen of calcium/vitamin D supplementation should be further studied and optimized in order that it safely helps mitigate anxiety and enhance memory in osteoporotic patients with certain psychiatric diseases (e.g., mood disorder).
Regarding the limitations of the present study, the exact explanation why only vitamin D3-treated group showed anxiolytic-like behavior is unclear, but it might be due to the effect of calcium supplement that could reduce the expression of VDR [78], thereby obscuring the positive effect of vitamin D3. In addition, female rats were not investigated in the present study; therefore, further experiments are required to confirm the present findings in female rats, which may somewhat have sex difference. For example, the intestinal absorptive cells of female rats often express higher calcium transporter protein levels (e.g., TRPV6 calcium channel) than those of male rats, which might, in turn, affect the response of female rats to oral calcium supplement. Moreover, future determinations of BrdU incorporation and biomarkers of mature hippocampal neuron [e.g., hexaribonucleotide binding protein-3 (NeuN) and prospero homeobox protein 1 (Prox1) in dentate gyrus] in calcium/vitamin D3-supplemented rats are worth exploring.
Conclusions
The 4-week calcium and/or vitamin D supplementation were demonstrated to help alleviate anxiety and enhance recognition memory in rats, as evaluated by EPM and NOR tests, respectively. Although the anxiolytic-like action of calcium supplement was not detected by EPM, it was able to enhance locomotor activity as determined by OFT. Both calcium and vitamin D also upregulated hippocampal DCX protein expression, which was an indirect proxy indicator of neurogenesis, while having no significant effect on hippocampal cell apoptosis. An increase in DCX expression in the hippocampus was probably a mechanism to help protect brain against anxiety and to improve the hippocampus-dependent cognitive function. Nevertheless, it is important to note that inappropriate calcium supplementation (e.g., prolonged high-dose calcium supplement) may induce certain adverse outcomes, such as an increased risk of dementia [19]. Therefore, our findings have provided foundation for further development of better and safe calcium/vitamin D supplement regimens that help improve mood and memory for individuals with osteopenia, osteoporosis or fracture, agreeing with SDG 3 (Good Health and Well-being) of United Nations Sustainable Development Goals (SDG).
Supporting information
Original Western blot images of protein expression levels of hippocampal doublecortin (DCX) and β-actin in rats supplemented with calcium and/or vitamin D3 for 4 weeks.
(PDF)
The tables show raw data of body weight, daily food and water intakes; serum and urinary calcium and serum 25(OH)D3; parameters measured by elevated plus-maze (EPM), open field test (OFT), and novel object recognition (NOR); and doublecortin expression and TUNEL-positive cells in hippocampus in control, calcium chloride (Ca), vitamin D3 (Vit D), and (Ca+Vit D) group.
(PDF)
Acknowledgments
We thank Prof. Nateetip Krishnamra for critical comments on the experimental data and English language editing, and Thitapha Kiattisirichai for the artworks (Figs 3A, 4A and 5A).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from RGJ Advanced Program, Thailand Science Research and Innovation (TSRI; RAP61K0020 to SL), the Research Promotion and Development Office, Siam University (007/05/2561 to SL), Faculty of Medicine, Siam University (MEDSIAM RESEACH 01/2561 to SL), and Faculty of Medicine, Thammasat University (to JC). N. Charoenphandhu is NRCT Distinguished Research Professor awarded by National Research Council of Thailand (NRCT)—Mahidol University, and TSRI/Mahidol University [Fundamental Fund: fiscal year 2022 and 2023 by National Science Research and Innovation Fund (NSRF)]. There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34(1):47–64. doi: 10.1016/j.yfrne.2012.07.001 . [DOI] [PubMed] [Google Scholar]
- 2.Latimer CS, Brewer LD, Searcy JL, Chen KC, Popovic J, Kraner SD, et al. Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc Natl Acad Sci U S A. 2014;111(41):E4359–66. doi: 10.1073/pnas.1404477111 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohmann C. Calcium signaling and the development of specific neuronal connections. Prog Brain Res. 2009;175:443–52. doi: 10.1016/S0079-6123(09)17529-5 . [DOI] [PubMed] [Google Scholar]
- 4.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670–6. doi: 10.1056/NEJM199709043371003 . [DOI] [PubMed] [Google Scholar]
- 5.Fardellone P, Brazier M, Kamel S, Gueris J, Graulet AM, Lienard J, et al. Biochemical effects of calcium supplementation in postmenopausal women: influence of dietary calcium intake. Am J Clin Nutr. 1998;67(6):1273–8. doi: 10.1093/ajcn/67.6.1273 . [DOI] [PubMed] [Google Scholar]
- 6.Gallagher JC, Goldgar D. Treatment of postmenopausal osteoporosis with high doses of synthetic calcitriol. A randomized controlled study. Ann Intern Med. 1990;113(9):64–55. doi: 10.7326/0003-4819-113-9-649 . [DOI] [PubMed] [Google Scholar]
- 7.Williamson L, Hayes A, Hanson ED, Pivonka P, Sims NA, Gooi JH. High dose dietary vitamin D3 increases bone mass and strength in mice. Bone Rep. 2017;6:44–50. doi: 10.1016/j.bonr.2017.02.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wongdee K, Chanpaisaeng K, Teerapornpuntakit J, Charoenphandhu N. Intestinal calcium absorption. Compr. Physiol. 2021;11(3):2047–73. doi: 10.1002/cphy.c200014 . [DOI] [PubMed] [Google Scholar]
- 9.Liel Y, Ulmer E, Shary J, Hollis BW, Bell NH. Low circulating vitamin D in obesity. Calcif Tissue Int. 1988;43(4):199–201. doi: 10.1007/BF02555135 . [DOI] [PubMed] [Google Scholar]
- 10.Salehpour A, Hosseinpanah F, Shidfar F, Vafa M, Razaghi M, Dehghani S, et al. A 12-week double-blind randomized clinical trial of vitamin D3 supplementation on body fat mass in healthy overweight and obese women. Nutr J. 2012;11:78. doi: 10.1186/1475-2891-11-78 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veale WL, Myers RD. Emotional behavior, arousal and sleep produced by sodium and calcium ions perfused within the hypothalamus of the cat. Physiol Behav. 1971;7(4):601–7. doi: 10.1016/0031-9384(71)90115-6 . [DOI] [PubMed] [Google Scholar]
- 12.Brown SW, Vyas BV, Spiegel DR. Mania in a case of hyperparathyroidism. Psychosomatics. 2007;48(3):265–8. doi: 10.1176/appi.psy.48.3.265 . [DOI] [PubMed] [Google Scholar]
- 13.Burne TH, Feron F, Brown J, Eyles DW, McGrath JJ, Mackay-Sim A. Combined prenatal and chronic postnatal vitamin D deficiency in rats impairs prepulse inhibition of acoustic startle. Physiol Behav. 2004;81(4):651–5. doi: 10.1016/j.physbeh.2004.03.004 . [DOI] [PubMed] [Google Scholar]
- 14.Groves NJ, Kesby JP, Eyles DW, McGrath JJ, Mackay-Sim A, Burne TH. Adult vitamin D deficiency leads to behavioural and brain neurochemical alterations in C57BL/6J and BALB/c mice. Behav Brain Res. 2013;241:120–31. doi: 10.1016/j.bbr.2012.12.001 . [DOI] [PubMed] [Google Scholar]
- 15.Godinho AF, Trombini TV, Oliveira EC. Effects of elevated calcium on motor and exploratory activities of rats. Braz J Med Biol Res. 2002;35(4):451–7. doi: 10.1590/s0100-879x2002000400007 . [DOI] [PubMed] [Google Scholar]
- 16.Trulson ME, Arasteh K, Ray DW. Effects of elevated calcium on learned helplessness and brain serotonin metabolism in rats. Pharmacol Biochem Behav. 1986;24(3):445–8. doi: 10.1016/0091-3057(86)90539-3 . [DOI] [PubMed] [Google Scholar]
- 17.Koshkina A, Dudnichenko T, Baranenko D, Fedotova J, Drago F. Effects of vitamin D3 in long-term ovariectomized rats subjected to chronic unpredictable mild stress: BDNF, NT-3, and NT-4 implications. Nutrients. 2019;11(8). doi: 10.3390/nu11081726 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki M, Yoshioka M, Hashimoto M, Murakami M, Noya M, Takahashi D, et al. Randomized, double-blind, placebo-controlled trial of vitamin D supplementation in Parkinson disease. Am J Clin Nutr. 2013;97(5):1004–13. doi: 10.3945/ajcn.112.051664 . [DOI] [PubMed] [Google Scholar]
- 19.Kern J, Kern S, Blennow K, Zetterberg H, Waern M, Guo X, et al. Calcium supplementation and risk of dementia in women with cerebrovascular disease. Neurology. 2016;87(16):1674–80. doi: 10.1212/WNL.0000000000003111 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeCarolis NA, Eisch AJ. Hippocampal neurogenesis as a target for the treatment of mental illness: a critical evaluation. Neuropharmacology. 2010;58(6):884–93. doi: 10.1016/j.neuropharm.2009.12.013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy MJF, Boulle F, Steinbusch HW, van den Hove DLA, Kenis G, Lanfumey L. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology (Berl). 2018;235(8):2195–220. doi: 10.1007/s00213-018-4950-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33(1):88–109. doi: 10.1038/sj.npp.1301574 . [DOI] [PubMed] [Google Scholar]
- 23.Groves NJ, Bradford D, Sullivan RK, Conn KA, Aljelaify RF, McGrath JJ, et al. Behavioural effects of adult vitamin D deficiency in BALB/c mice are not associated with proliferation or survival of neurons in the adult hippocampus. PLoS One. 2016;11(4):e0152328. doi: 10.1371/journal.pone.0152328 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toth AB, Shum AK, Prakriya M. Regulation of neurogenesis by calcium signaling. Cell Calcium. 2016;59(2–3):124–34. doi: 10.1016/j.ceca.2016.02.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Y, Zhou R, Yang R, Zhang Z, Bai Y, Chang F, et al. Abnormal neurogenesis in the dentate gyrus of adult mice lacking 1,25-dihydroxy vitamin D3 (1,25-(OH)2D3). Hippocampus. 2012;22(3):421–33. doi: 10.1002/hipo.20908 . [DOI] [PubMed] [Google Scholar]
- 26.Hill AS, Sahay A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology. 2015;40(10):2368–78. doi: 10.1038/npp.2015.85 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milosevic J, Juch F, Storch A, Schwarz J. Low extracellular calcium is sufficient for survival and proliferation of murine mesencephalic neural precursor cells. Cell Tissue Res. 2006;324(3):377–84. doi: 10.1007/s00441-005-0147-3 . [DOI] [PubMed] [Google Scholar]
- 28.Van Cromphaut SJ, Rummens K, Stockmans I, Van Herck E, Dijcks FA, Ederveen AG, et al. Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms. J Bone Miner Res. 2003;18(10):1725–36. doi: 10.1359/jbmr.2003.18.10.1725 . [DOI] [PubMed] [Google Scholar]
- 29.Jolles JW, Boogert NJ, van den Bos R. Sex differences in risk-taking and associative learning in rats. R Soc Open Sci. 2015;2(11):150485. doi: 10.1098/rsos.150485 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shepherd JK, Flores T, Rodgers RJ, Blanchard RJ, Blanchard DC. The anxiety/defense test battery: influence of gender and ritanserin treatment on antipredator defensive behavior. Physiol Behav. 1992;51(2):277–85. doi: 10.1016/0031-9384(92)90141-n . [DOI] [PubMed] [Google Scholar]
- 31.Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19(2):234–46. doi: 10.1111/j.0953-816x.2003.03123.x . [DOI] [PubMed] [Google Scholar]
- 32.Plümpe T, Ehninger D, Steiner B, Klempin F, Jessberger S, Brandt M, et al. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006;7:77. doi: 10.1186/1471-2202-7-77 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heffner TG, Hartman JA, Seiden LS. A rapid method for the regional dissection of the rat brain. Pharmacol Biochem Behav. 1980;13(3):453–6. doi: 10.1016/0091-3057(80)90254-3 . [DOI] [PubMed] [Google Scholar]
- 34.Vnek N, Rothblat LA. The hippocampus and long-term object memory in the rat. J Neurosci. 1996;16(8):2780–7. doi: 10.1523/JNEUROSCI.16-08-02780.1996 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suntornsaratoon P, Krishnamra N, Charoenphandhu N. Positive long-term outcomes from presuckling calcium supplementation in lactating rats and the offspring. Am J Physiol Endocrinol Metab. 2015;308(11):E1010–22. doi: 10.1152/ajpendo.00049.2015 . [DOI] [PubMed] [Google Scholar]
- 36.Vieth R. Why the optimal requirement for Vitamin D3 is probably much higher than what is officially recommended for adults. J Steroid Biochem Mol Biol. 2004;89–90(1–5):575–9. doi: 10.1016/j.jsbmb.2004.03.038 . [DOI] [PubMed] [Google Scholar]
- 37.Detregiachi C, Santos Bueno P, Barbalho S, Quesada K, Brandão C, Pedroso I, et al. Effect of vitamin D supplements on the body weight and glycaemia in Wistar rats. J Pharm Res Int. 2016;10(1):1–8. doi: 10.9734/BJPR/2016/23508 [DOI] [Google Scholar]
- 38.Dornellas APS, Boldarine VT, Pedroso AP, Carvalho LOT, de Andrade IS, Vulcani-Freitas TM, et al. High-fat feeding improves anxiety-type behavior induced by ovariectomy in rats. Front Neurosci. 2018;12:557. doi: 10.3389/fnins.2018.00557 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao RM, Sadananda M. Influence of state and/or trait anxieties of wistar rats in an anxiety paradigm. Ann Neurosci. 2016;23(1):44–50. doi: 10.1159/000443555 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reamtong O, Lapmanee S, Tummatorn J, Palavong N, Thongsornkleeb C, Ruchirawat S. Synthesis of benzoazepine derivatives via azide rearrangement and evaluation of their antianxiety activities. ACS Med Chem Lett. 2021;12(9):1449–58. doi: 10.1021/acsmedchemlett.1c00275 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lapmanee S, Charoenphandhu N, Krishnamra N, Charoenphandhu J. Anxiolytic-like actions of reboxetine, venlafaxine and endurance swimming in stressed male rats. Behav Brain Res. 2012;231(1):20–8. doi: 10.1016/j.bbr.2012.02.037 . [DOI] [PubMed] [Google Scholar]
- 42.Lapmanee S, Charoenphandhu J, Teerapornpuntakit J, Krishnamra N, Charoenphandhu N. Agomelatine, venlafaxine, and running exercise effectively prevent anxiety- and depression-like behaviors and memory impairment in restraint stressed rats. PLoS One. 2017;12(11):e0187671. doi: 10.1371/journal.pone.0187671 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanahisa Y, Yamaguchi M. Characterization of calcium accumulation in the brain of rats administered orally calcium: the significance of energy-dependent mechanism. Mol Cell Biochem. 1996;158(1):1–7. doi: 10.1007/BF00225876 . [DOI] [PubMed] [Google Scholar]
- 44.Taylor EN, Curhan GC. Demographic, dietary, and urinary factors and 24-h urinary calcium excretion. Clin J Am Soc Nephrol. 2009;4(12):1980–7. doi: 10.2215/CJN.02620409 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taheri M, Tavasoli S, Shokrzadeh F, Amiri FB, Basiri A. Effect of vitamin D supplementation on 24-hour urine calcium in patients with calcium Urolithiasis and vitamin D deficiency. Int Braz J Urol. 2019;45(2):340–6. doi: 10.1590/S1677-5538.IBJU.2018.0522 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171(3):537–47. doi: 10.1083/jcb.200505155 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JW, Shin CY. Deciphering the role of T-type calcium channels in regulating adult hippocampal neurogenesis. Acta Physiol (Oxf). 2021;232(1):e13643. doi: 10.1111/apha.13643 . [DOI] [PubMed] [Google Scholar]
- 48.Alenina N, Klempin F. The role of serotonin in adult hippocampal neurogenesis. Behav Brain Res. 2015;277:49–57. doi: 10.1016/j.bbr.2014.07.038 . [DOI] [PubMed] [Google Scholar]
- 49.Brion F, Dupuis Y. Calcium and monoamine regulation: role of vitamin D nutrition. Can J Physiol Pharmacol. 1980;58(12):1431–4. doi: 10.1139/y80-217 . [DOI] [PubMed] [Google Scholar]
- 50.Brus R, Nowak P, Szkilnik R, Mikolajun U, Kostrzewa RM. Serotoninergics attenuate hyperlocomotor activity in rats. Potential new therapeutic strategy for hyperactivity. Neurotox Res. 2004;6(4):317–25. doi: 10.1007/BF03033442 . [DOI] [PubMed] [Google Scholar]
- 51.Chatterjee O, Nakchbandi IA, Philbrick WM, Dreyer BE, Zhang JP, Kaczmarek LK, et al. Endogenous parathyroid hormone-related protein functions as a neuroprotective agent. Brain Res. 2002;930(1–2):58–66. doi: 10.1016/s0006-8993(01)03407-2 . [DOI] [PubMed] [Google Scholar]
- 52.Weaver SR, Hernandez LL. Autocrine-paracrine regulation of the mammary gland. J Dairy Sci. 2016;99(1):842–53. doi: 10.3168/jds.2015-9828 . [DOI] [PubMed] [Google Scholar]
- 53.Xu M, Chen WQ, Wang JP, Foster D Jr., Xu DY. 5-HT3 receptors in the central amygdala mediate the modulation of thymus function in rats. Sheng Li Xue Bao. 2007;59(1):42–50. . [PubMed] [Google Scholar]
- 54.Ozawa M, Ninomiya T, Ohara T, Hirakawa Y, Doi Y, Hata J, et al. Self-reported dietary intake of potassium, calcium, and magnesium and risk of dementia in the Japanese: the Hisayama Study. J Am Geriatr Soc. 2012;60(8):1515–20. doi: 10.1111/j.1532-5415.2012.04061.x . [DOI] [PubMed] [Google Scholar]
- 55.Biancuzzo RM, Clarke N, Reitz RE, Travison TG, Holick MF. Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation. J Clin Endocrinol Metab. 2013;98(3):973–9. doi: 10.1210/jc.2012-2114 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spence LA, Cifelli CJ, Miller GD. The role of dairy products in healthy weight and body composition in children and adolescents. Curr Nutr Food Sci. 2011;7(1):40–9. doi: 10.2174/157340111794941111 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC. Regulation of adiposity by dietary calcium. FASEB J. 2000;14(9):1132–8. . [PubMed] [Google Scholar]
- 58.Marcotorchino J, Tourniaire F, Astier J, Karkeni E, Canault M, Amiot MJ, et al. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J Nutr Biochem. 2014;25(10):1077–83. doi: 10.1016/j.jnutbio.2014.05.010 . [DOI] [PubMed] [Google Scholar]
- 59.Tablas MB, Goto RL, Caetano BFR, dos Santos SAA, Barbisan LF. Vitamin D3 suppresses the early stages of chemically induced hepatocarcinogenesis in rats: a dose-response analysis. Nutrire. 2018;43. doi: 10.1186/s41110-018-0065-2 [DOI] [Google Scholar]
- 60.Sergeev IN. Vitamin D-cellular Ca2+ link to obesity and diabetes. J Steroid Biochem Mol Biol. 2016;164:326–30. doi: 10.1016/j.jsbmb.2015.11.008 . [DOI] [PubMed] [Google Scholar]
- 61.Tzotzas T, Papadopoulou FG, Tziomalos K, Karras S, Gastaris K, Perros P, et al. Rising serum 25-hydroxy-vitamin D levels after weight loss in obese women correlate with improvement in insulin resistance. J Clin Endocrinol Metab. 2010;95(9):4251–7. doi: 10.1210/jc.2010-0757 . [DOI] [PubMed] [Google Scholar]
- 62.Gauvin DV, Zimmermann ZJ, Dalton JA, Baird TJ, Kallman MJ. CNS safety screening under ICH S7A guidelines requires observations of multiple behavioral units to assess motor function. Int J Toxicol. 2019;38(5):339–56. doi: 10.1177/1091581819864836 . [DOI] [PubMed] [Google Scholar]
- 63.Holick MF. Vitamin D and brain health: the need for vitamin D supplementation and sensible sun exposure. J Intern Med. 2015;277(1):90–3. doi: 10.1111/joim.12308 . [DOI] [PubMed] [Google Scholar]
- 64.Jääskeläinen T, Knekt P, Suvisaari J, Männistö S, Partonen T, Sääksjärvi K, et al. Higher serum 25-hydroxyvitamin D concentrations are related to a reduced risk of depression. Br J Nutr. 2015;113(9):1418–26. doi: 10.1017/S0007114515000689 . [DOI] [PubMed] [Google Scholar]
- 65.Pan P, Jin DH, Chatterjee-Chakraborty M, Halievski K, Lawson D, Remedios D, et al. The effects of vitamin D3 during pregnancy and lactation on offspring physiology and behavior in sprague-dawley rats. Dev Psychobiol. 2014;56(1):12–22. doi: 10.1002/dev.21086 . [DOI] [PubMed] [Google Scholar]
- 66.Fedotova JO. Vitamin D3 treatment differentially affects anxiety-like behavior in the old ovariectomized female rats and old ovariectomized female rats treated with low dose of 17β-estradiol. BMC Med Genet. 2019;20(Suppl 1):49. doi: 10.1186/s12881-019-0774-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mayne PE, Burne THJ. Vitamin D in synaptic plasticity, cognitive function, and neuropsychiatric illness. Trends Neurosci. 2019;42(4):293–306. doi: 10.1016/j.tins.2019.01.003 . [DOI] [PubMed] [Google Scholar]
- 68.Steardo L Jr., Luciano M, Sampogna G, Carbone EA, Caivano V, Di Cerbo A, et al. Clinical severity and calcium metabolism in patients with bipolar disorder. Brain Sci. 2020;10(7). doi: 10.3390/brainsci10070417 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaneko I, Sabir MS, Dussik CM, Whitfield GK, Karrys A, Hsieh JC, et al. 1,25-Dihydroxyvitamin D regulates expression of the tryptophan hydroxylase 2 and leptin genes: implication for behavioral influences of vitamin D. FASEB J. 2015;29(9):4023–35. doi: 10.1096/fj.14-269811 . [DOI] [PubMed] [Google Scholar]
- 70.Fazelian S, Amani R, Paknahad Z, Kheiri S, Khajehali L. Effect of vitamin D supplement on mood status and inflammation in vitamin D deficient type 2 diabetic women with anxiety: A randomized clinical trial. Int J Prev Med. 2019;10:17. doi: 10.4103/ijpvm.IJPVM_174_18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Groves NJ, Burne THJ. The impact of vitamin D deficiency on neurogenesis in the adult brain. Neural Regen Res. 2017;12(3):393–4. doi: 10.4103/1673-5374.202936 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liang Q, Cai C, Duan D, Hu X, Hua W, Jiang P, et al. Postnatal vitamin D intake modulates hippocampal learning and memory in adult mice. Front Neurosci. 2018;12:141. doi: 10.3389/fnins.2018.00141 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang H, Li Q, Tang H, Ding J, Xu N, Sun S, et al. The activated newborn neurons participate in enriched environment induced improvement of locomotor function in APP/PS1 mice. Brain Behav. 2019;9(7):e01316. doi: 10.1002/brb3.1316 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hasegawa Y, Inoue T, Fuji T. Calcium carbonate supplementation causes memory impairment in mice. Asian Pac J Trop Med. 2018;11:576–82. doi: 10.4103/1995-7645.244522 [DOI] [Google Scholar]
- 75.Mazahery H, von Hurst PR. Factors affecting 25-hydroxyvitamin D concentration in response to vitamin D supplementation. Nutrients. 2015;7(7):5111–42. doi: 10.3390/nu7075111 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Letavernier E, Verrier C, Goussard F, Perez J, Huguet L, Haymann JP, et al. Calcium and vitamin D have a synergistic role in a rat model of kidney stone disease. Kidney Int. 2016;90(4):809–17. doi: 10.1016/j.kint.2016.05.027 . [DOI] [PubMed] [Google Scholar]
- 77.Malihi Z, Wu Z, Stewart AW, Lawes CM, Scragg R. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104(4):1039–51. doi: 10.3945/ajcn.116.134981 . [DOI] [PubMed] [Google Scholar]
- 78.Conceição EP, Moura EG, Manhães AC, Carvalho JC, Nobre JL, Oliveira E, et al. Calcium reduces vitamin D and glucocorticoid receptors in the visceral fat of obese male rats. J Endocrinol. 2016;230(2):263–74. doi: 10.1530/JOE-16-0041 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Original Western blot images of protein expression levels of hippocampal doublecortin (DCX) and β-actin in rats supplemented with calcium and/or vitamin D3 for 4 weeks.
(PDF)
The tables show raw data of body weight, daily food and water intakes; serum and urinary calcium and serum 25(OH)D3; parameters measured by elevated plus-maze (EPM), open field test (OFT), and novel object recognition (NOR); and doublecortin expression and TUNEL-positive cells in hippocampus in control, calcium chloride (Ca), vitamin D3 (Vit D), and (Ca+Vit D) group.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.