Figure 1. Photo-affinity chromatography of labile therapeutics.
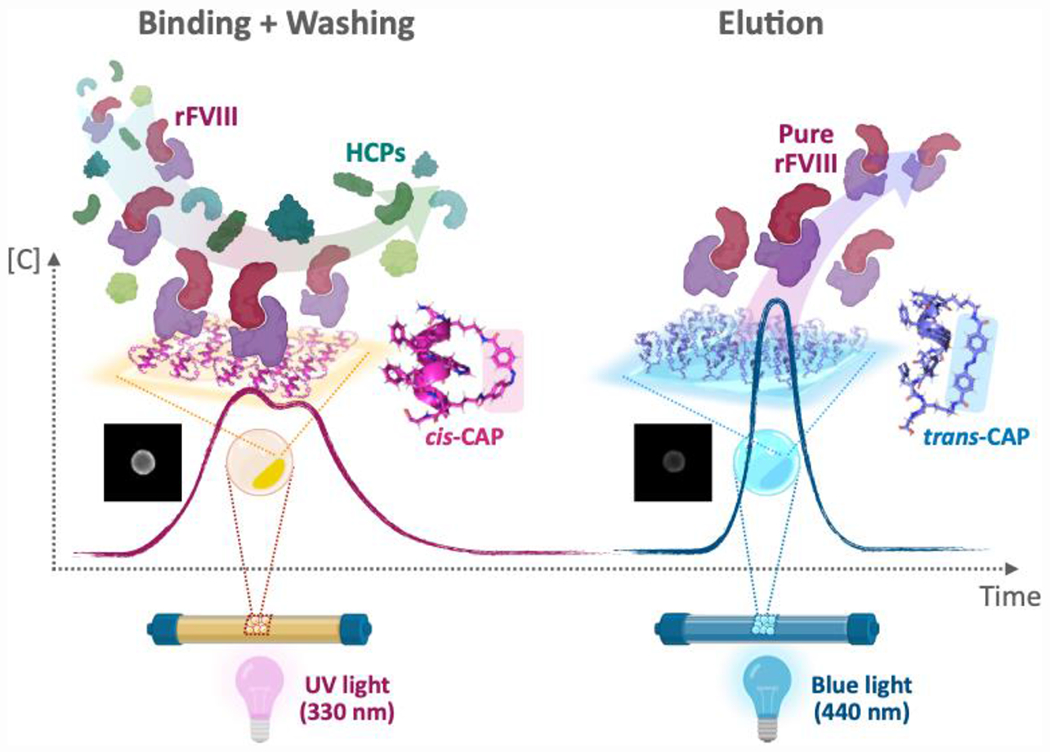
The target recombinant human blood coagulation Factor VIII (rVIII) – a labile therapeutic protein – is captured by the CAPs displayed on translucent ChemMatrix beads, while the impurities are cleared, and subsequently released at high purity under benign conditions (< 10 mW·cm−2). Specifically, (i) the pore surface of the beads is functionalized with cyclic azobenzene-peptides (CAPs), each comprising a peptide segment, which interacts with the target rFVIII, and a photo-responsive azobenzene linker, which switches reversibly the peptide segment between a rFVIII-binding (cis) and a rFVIII-releasing (trans) conformation; (ii) when rFVIII in solution contacts the beads, it diffuses into their pores, and reaches the inner surface of the CAP-functionalized beads, where it is captured by the affinity binding of cis-CAPs § (note: the CAPs developed in this study mainly target the A1 domain of rFVIII); conversely, the other species present in the feedstock do not interact with the CAPs and are therefore removed by washing; (iii) following the adsorption of rFVIII, the beads are washed and exposed to blue light (440 nm) to isomerize cis-CAPs into trans-CAPs, thus triggering the dissociation of the rFVIII:CAP complex and the release of rFVIII from the resin beads; finally, (iv) the beads can subsequently be exposed to UV light (330 nm), which switches the trans-CAPs back into cis-CAPs, thus preparing the beads for a subsequent round of photo-affinity purification of rFVIII.
