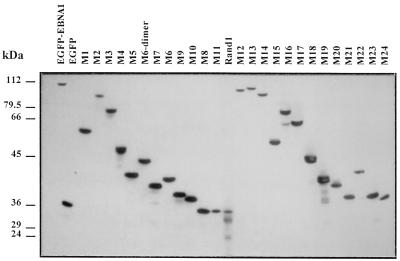
FIG. 4.
Immunoblot analysis of various EBNA-1 mutants fused to EGFP. Expression of EBNA-1 derivatives fused to EGFP was analyzed by Western blotting in transiently transfected HeLa cells. In this experiment, HeLa cells were 24 to 28% transfected, as shown by fluorescence-activated cell sorter analysis. Total proteins were separated by SDS-polyacrylamide gel electrophoresis (20 μg per lane), transferred on nitrocellulose, and incubated with a rabbit serum directed against GFP. Detection was performed by chemiluminescence. The relative mobility of the fusion proteins and the absence of free GFP were compatible with the absence of major proteolytic degradation, except for mutants M19 and Rand1, which showed moderate degradation. Mutant Rand2 (data not shown) and mutant M11, which contained the same amino acids as Rand1 in a different order, did not show any significant proteolysis. Note that the average expression level of the various EGFP EBNA-1 derivatives is unrelated to their ability to associate with metaphase chromosomes.