Fig. 3. Tumor CGP complements clinically informed germline genetic testing referral.
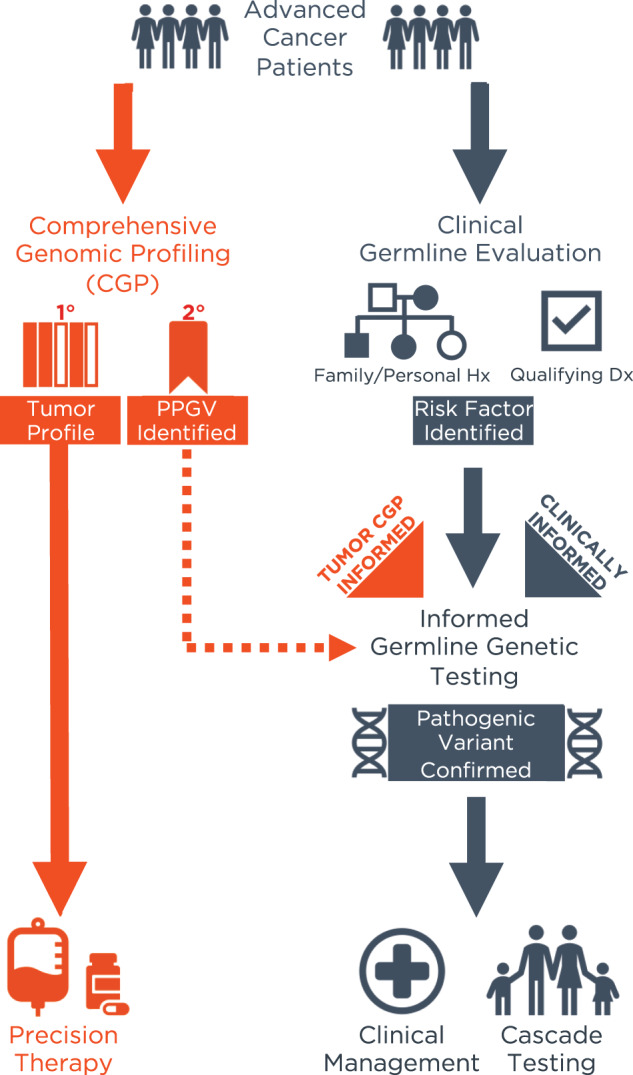
In current practice, referral for germline testing is cancer type-dependent, pursued when clinically indicated due to identification of risk factors upon initial clinical germline evaluation, if performed, and/or in accordance with established guidelines for the tumor type. While the primary purpose of tumor CGP is to inform clinical decision-making regarding cancer treatment, secondary finding of PPGVs can complement clinical germline evaluation in identifying patients who should be referred for germline genetic testing when it may not otherwise be considered with potential implications for clinical management and familial genetic risk assessment. PPGV Potential Pathogenic Germline Variant.
