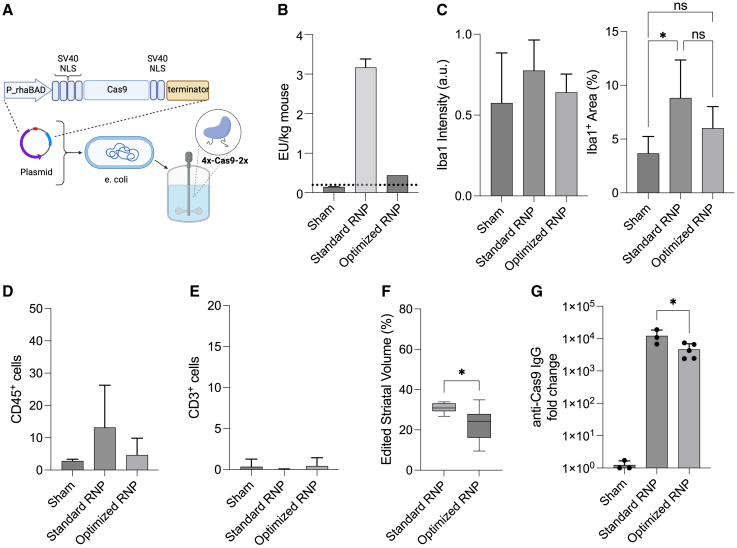
Figure 3.
Optimized, low endotoxin RNP formulation reduces local immune response
(A) Schematic of manufacturing scale-up to produce industrial ultra-low endotoxin 4x-SpyCas9-2x protein using a tag-free expression and purification system. (B) Endotoxin levels calculated on a per mouse basis between the standard (laboratory 4x-SpyCas9-2x with sg298 2018) and optimized (industrial 4x-SpyCas9-2x protein with sg298 2022) RNP formulations at 25 M measured by LAL assay. Dotted line indicates FDA recommendation of 0.2 EU/kg/h for drug products administered intrathecally in humans. (C) Quantification of Iba1+ staining intensity and percent area (n = 6–10, one-way ANOVA, ∗p < 0.05). (D) Quantification of CD45+ and (E) CD3+ cells per image (n = 6–10, one-way ANOVA, ns). (F) Percent volume of edited striatal tissue for Cas9 RNPs injected at 25 M (n = 6–10 injections). (G) Quantification of IgG antibodies against Cas9 or AAV capsid proteins measured 21 days after bilateral intrastriatal injections by ELISA (n = 3–5 biological replicates).