Table 3.
Summary of eHealth intervention effects on abstinence by intervention type and follow-up, based on GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) guidelines.
| Outcome and follow-up | Summary of the effect | Number of participants and studies | Quality of the evidence (GRADE)a | Summary for intervention | ||||||
| Smokers with intention to quit (by follow-up) | ||||||||||
|
|
High-frequency SMS or app text messaging versus low-frequency SMS or app text messaging | |||||||||
|
|
|
3 months | Log RRb=−0.01, 95% CI −0.25 to 0.28; I2=38.77%; Little or no increasec | 8958 participants; 2 studies |
 d,e; Low d,e; Low |
May make little or no increase on cessation | ||||
|
|
|
6 months | Log RR=0.00, 95% CI −0.07 to 0.08; I2=0.46%; Little or no increase | 8958 participants; 2 studies |
 d,e; Low d,e; Low |
May make little or no increase on cessation | ||||
|
|
SMS or app text messaging versus minimal smoking cessation support | |||||||||
|
|
|
3 months | Log RR=0.50, 95% CI 0.25 to 0.75; I2=0.72%; Moderate increase | 1367 participants; 5 studies |
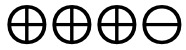 d; Moderate d; Moderate |
Probably increase cessation moderately | ||||
|
|
|
6 months | Log RR=0.77, 95% CI 0.49 to 1.04; I2=8.65%; Important increase | 1153 participants; 3 studies |
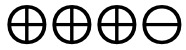 d; Moderate d; Moderate |
Probably increase cessation significantly | ||||
|
|
mHealthg app versus less intensive smoking cessation support | |||||||||
|
|
|
3 months | Log RR=0.76, 95% CI 0.09 to 1.42; I2=88.02%; Important increase | 1167 participants; 4 studies |
 d,f; Low d,f; Low |
May increase cessation significantly | ||||
|
|
|
6 months | Log RR=0.15, 95% CI −0.18 to 0.48; I2=80.06%; Little or no increase | 9360 participants; 6 studies |
 e,f; Low e,f; Low |
May make little or no increase on cessation | ||||
|
|
mHealth app + psycho or pharmacological therapy versus psycho or pharmacological therapy | |||||||||
|
|
|
6 months | Log RR=0.25, 95% CI −0.18 to 0.67; I2=16.91%; Little or no increase | 340 participants; 2 studies |
 e,f; Low e,f; Low |
May make little or no increase on cessation | ||||
| Smokers of special population (any follow-up) | ||||||||||
|
|
Adult smokers with mental disorders | Log RR=−0.25, 95% CI −1.92 to 1.42; I2=72.32%; Little or no increase | 813 participants; 3 studies |
 e,f; Low e,f; Low |
May make little or no increase on cessation | |||||
|
|
Hospitalized adult smokers | Log RR=1.00, 95% CI 0.22 to 1.78; I2=3.45%; Important increase | 466 participants; 2 studies |
 d,e; Low d,e; Low |
May increase cessation significantly | |||||
|
|
Pregnant smokers (including adolescents) | Log RR=0.34, 95% CI −0.01 to 0.68; I2=25.84%; Little or no increase | 2319 participants; 5 studies |
 e,h; Very low e,h; Very low |
May make little or no increase on cessation | |||||
aGRADE Working Group grades of evidence. 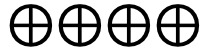 High quality: The authors have a lot of confidence that the true effect is similar to the estimated effect.
High quality: The authors have a lot of confidence that the true effect is similar to the estimated effect. 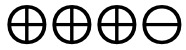 Moderate quality: The authors believe that the true effect is probably close to the estimated effect.
Moderate quality: The authors believe that the true effect is probably close to the estimated effect.  Low certainty: The true effect might be markedly different from the estimated effect. Very low certainty: The true effect is probably markedly different from the estimated effect [66].
Low certainty: The true effect might be markedly different from the estimated effect. Very low certainty: The true effect is probably markedly different from the estimated effect [66].
bRR: risk ratio.
cThe italicization serves as an abstract description of the effect size based on the 95% CI: 95% CI crosses 0=little or no increase, 95% CI does not cross 0 nor 1=moderate increase, and 95% CI does not cross 0 but cross 1=important increase.
dDowngraded 1 level for significant risk of bias: one study was rated as high risk of bias (2 unclear risk of bias count as one high risk of bias).
eDowngraded 1 level for imprecision: CIs encompass both clinically significant harm and clinically significant benefit, or fewer than 500 participants overall.
fDowngraded 1 level of inconsistency: considerable unexplained statistical heterogeneity (I2>50%).
gmHealth: mobile health.
hDowngraded 2 levels for serious risk of bias: 2 or more studies rated as high risk of bias (2 unclear risk of bias count as one high risk of bias).
