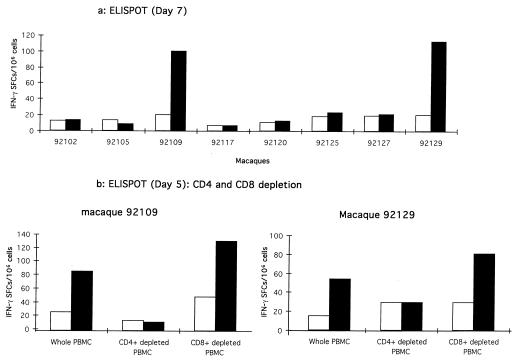
FIG. 1.
Synthesis of IFN-γ by CD4+ lymphocytes after LP-TT stimulation in macaques 92109 and 92129. (a) Helper peptide-specific IFN-γ SFCs in PBMCs from the eight macaques were analyzed after the third mixed-micelle lipopeptide immunization. The ELISPOT assay was performed 7 days after one short in vitro stimulation. Briefly, PBMCs (2.5 × 106/ml) were cultured for 3 days in 24-well microtiter plates (Costar, Cambridge, Mass.) in complete medium, with 5 μM LP-TT or with 5 μM irrelevant LP (Nef HIV 66-97) (LP-i). IL-2 (Boehringer, Mannheim, Germany) was then added to each well (10 IU/ml), and incubation was continued for 4 days. Effector cells were then washed, counted, and seeded in duplicate in 96-well nitrocellulose plates (Multi-Screen HA; Millipore, Bedford, Mass.) that had been coated with the mouse anti-human IFN-γ capture monoclonal antibody (Genzyme, Russelheim, Germany) (8 μg/ml in carbonate buffer) and blocked with complete medium. Effector cells were incubated at 105 and 2 × 105 per well with 5 μM LP-i (white columns) or 5 μM LP-TT (black columns) for 48 h in complete medium containing 20 IU of IL-2/ml. The ELISPOT assay was performed as previously described (33). Responses were considered significant if there were a minimum of five SFCs per well and if this number was at least twice that obtained with the negative control. (b) A depletion assay was done using anti-CD4 or anti-CD8 monoclonal antibodies (MAbs) on the Th cell responder macaques (90109 and 92129) to obtain CD4+-depleted PBMCs and CD8+-depleted PBMCs, together with whole PBMCs. PBMCs were incubated for 30 min with cocktails of anti-human CD8 (DAKO, Glostrup, Denmark; Becton Dickinson, Mountain View, Calif.; and Ortho Diagnostic Systems, Raritan, N.J.) (1 μl of each/106 cells) or cocktails of human anti-CD4 (DAKO; Sigma Chemical Co., St. Louis, Mo.; and Ortho Diagnostic Systems) (1 μl of each/106 cells) MAbs coated on Dynabeads in 500 μl of complete medium on ice (BioMag goat anti-mouse immunoglobulin G; PerSeptive Biosystems, Framingham, Mass.). Conjugate-coated cells were then removed with a magnet (Dynal) and cultured in vitro for 5 days. Finally, they were tested in the ELISPOT assay.