Figure 1. Study design for vaccination and both CHMI phases.
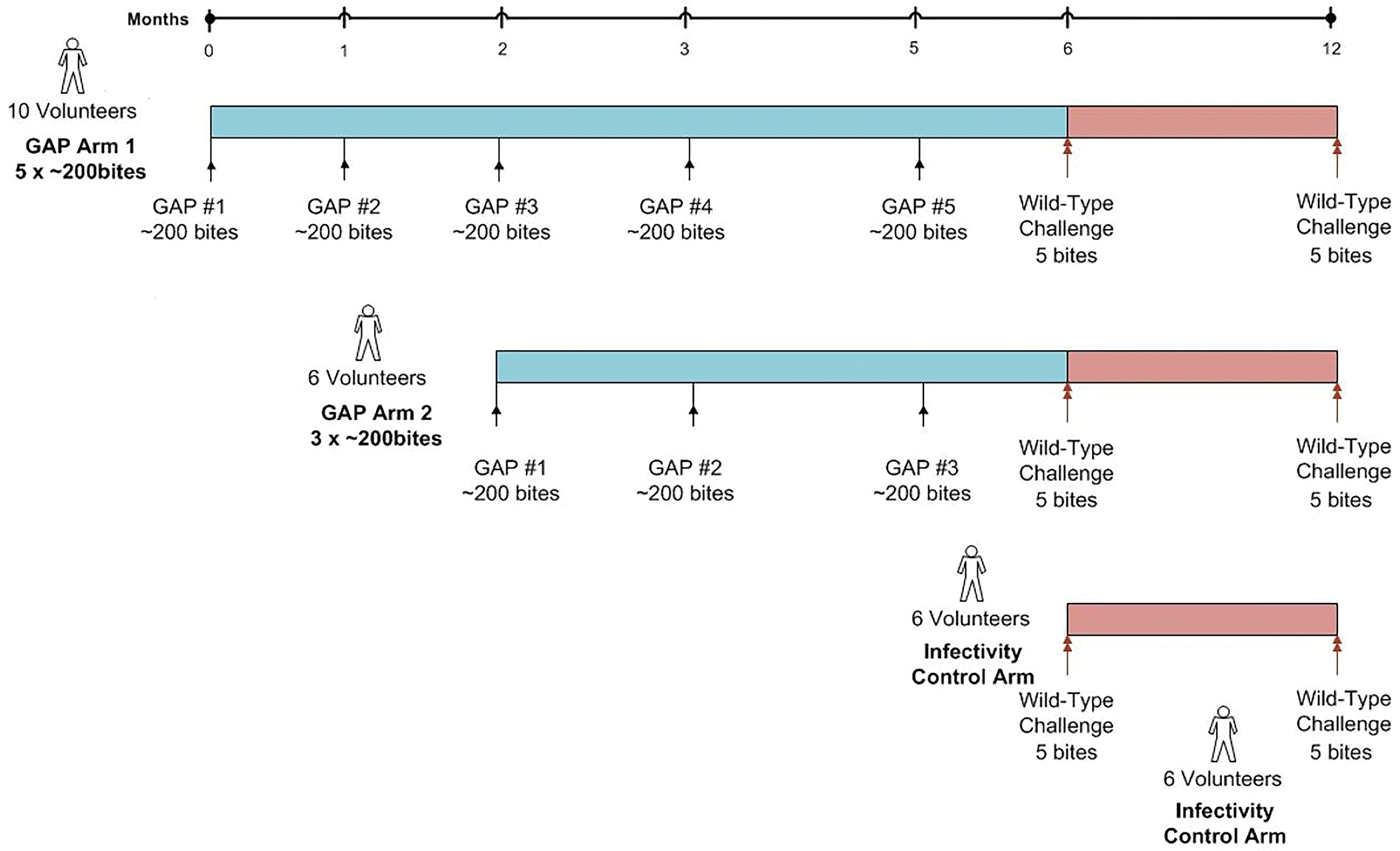
PfGAP3KO sporozoites were delivered to eligible study participants five (Aim 1) or three times (Arm 2) via ~200 PfGAP3KO infected mosquito bites per administration. The interval between doses was one month, except for the interval between the last two doses, which was two months. One month after the last immunization, vaccinated study participants and a group of malaria-naïve study participants as infectivity controls underwent CHMI induced by five wild-type Pf NF54-infected mosquito bites. Vaccinated study participants who were protected against the first CHMI were eligible for a second CHMI six months later, and these study participants were accompanied at CHMI by another group of malaria-naïve study participants as infectivity controls.
