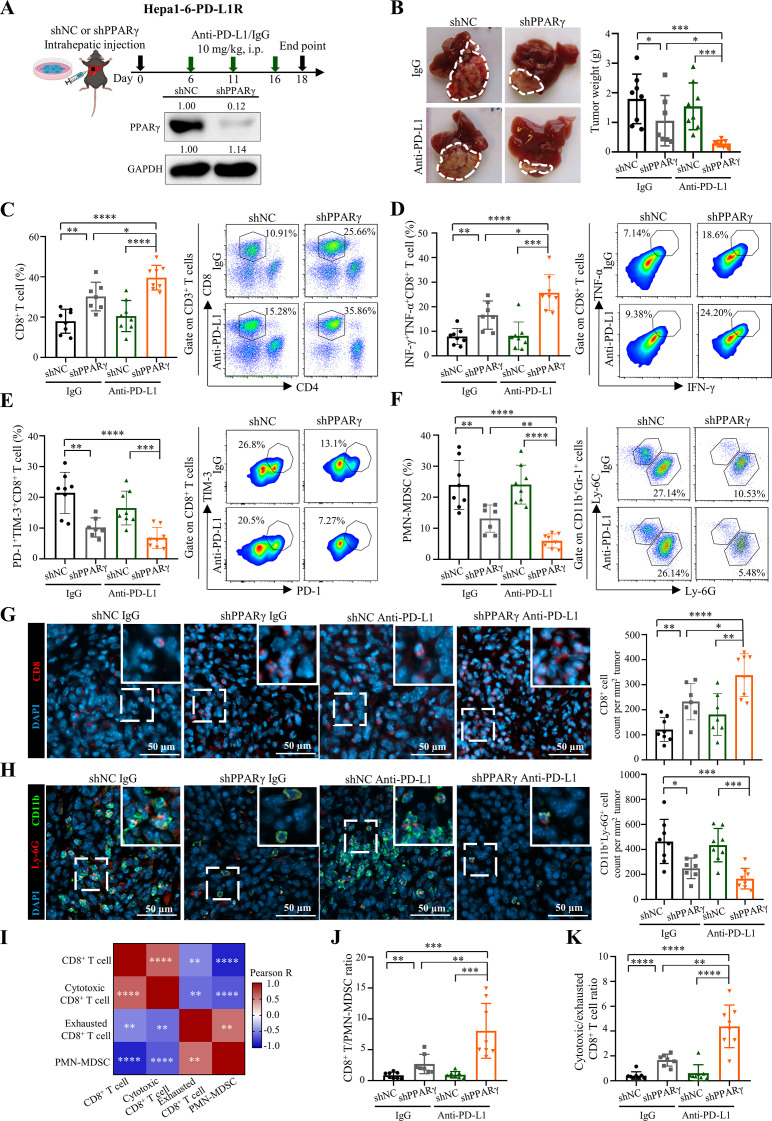
Figure 3.
Tumour-intrinsic PPARγ orchestrates TME remodelling to resist ICB therapy. (A) Treatment schedule for anti-PD-L1 antibody (10F.9G2) or isotype control (LTF-2) in Hepa1-6-PD-L1R-short hairpin RNA against negative control sequence (shNC)-tumour or shPPARγ-tumour bearing mice. In brief, 5×106 tumour cells were intrahepatically injected into C57BL/6 mice, which were then treated with anti-PD-L1 or isotype control antibodies via i.p. injection at day 6, 11 and 16. Tumour samples were collected at day 18-post tumour implantation for further analysis. Representative western blot images of PPARγ in Hepa1-6-PD-L1R-shNC or -shPPARγ stable cell lines are shown. GAPDH served as loading control. (B) Representative liver tumour photos and tumour weights of PD-L1R-shNC-tumour or shPPARγ-tumour bearing mice with treatment of anti-PD-L1 or isotype control at the endpoint are shown (n=7 to 8). (C) Representative flow cytometry dot plots and proportions of CD8+ T cells in CD45+ cells, (D) IFN-γ+TNF-α+ cells and (E) PD-1+TIM-3+ cells in CD8+ T cells, as well as (F) PMN-MDSCs in CD45+ cells in tumours from indicated groups are shown (n=7 to 8). (G) Representative immunofluorescence images of CD8 (red), (H) CD11b (green)/Ly-6G (red) and quantification dot plot bar graphs in liver tumours from indicated groups (n=7 to 8). DAPI (blue) indicates the nuclei staining. Scale bars, 50 µm. (I) Correlation heatmap of immune cell proportions calculated by data from C to F. (J) The ratios of CD8+ T/PMN-MDSCs and (K) cytotoxic/exhausted CD8+ T cell in tumours (n=7 to 8). Data represent as mean±SD. Statistical significance was determined by unpaired two-tailed Student’s t-test. Two-tailed Pearson’s correlation was used to describe the correlation between variables. *P<0.05; **p<0.01; ***p<0.001; ****p<0.0001. ICB, immune-checkpoint blockade; IFN, interferon; MDSC, myeloid-derived suppressor cell; PD-L1, programmed death-1-ligand-1; PMN, polymorphonuclear; PPARγ, peroxisome proliferator-activated receptor-gamma; TIM, T cell immunoglobulin; TME, tumour microenvironment; TNF, tumour necrosis factor.