Abstract
Introduction
Pregnancy in patients with chronic intestinal failure (CIF) is a relatively rare occurrence but is an important contemporary topic given both the increasing use of home parenteral nutrition (HPN) and the demographics of patients with CIF.
Method
An opinion-based survey was produced in a multidisciplinary manner, which was then distributed internationally, via the European Society for Clinical Nutrition and Metabolism network, using a web-based survey tool for healthcare professionals with a specialist interest in the management of CIF.
Results
Seventy specialists from 11 countries completed the survey. Fifty-four per cent of the respondents reported some experience of managing pregnancy in patients with CIF. However, 60% stated that they did not feel that it was their role to discuss the topic of pregnancy with their patients, with fewer than 10% stating that they routinely did so. Respondents felt that an individualised approach was required when considering alterations to parenteral support prior to conception, during pregnancy and in the postnatal period. Most respondents also felt there was no increased risk of catheter-related blood stream infections, while catheter-related thrombosis was deemed to be the most significant HPN-related complication for pregnant women.
Conclusion
This study reports a variable experience, knowledge and confidence of healthcare professionals when considering pregnancy in patients with CIF. The risk of HPN-related complication was felt to be greater during pregnancy, with an individualised approach being the preferred route for most aspects of care. The findings support the need for an international registry and subsequent consensus guidelines for the management of pregnancy in CIF.
Keywords: INTESTINAL FAILURE, PARENTERAL NUTRITION
WHAT IS ALREADY KNOWN ON THIS TOPIC
The diagnosis of intestinal failure in women of childbearing age is becoming increasingly encountered. There is currently limited experience of pregnancy in patients with chronic intestinal failure and as such there are no internationally agreed consensus statements or agreed guidelines.
WHAT THIS STUDY ADDS
With this study, we have demonstrated the existing experience and opinions related to the management of pregnancy in patients with chronic intestinal failure, including those related to pre-pregnancy counselling, management of parenteral nutrition in the trimesters of pregnancy, mode of delivery and the postnatal period.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The limited experience demonstrated by our study would indicate the need for an international registry, thus allowing for the development of consensus guidelines.
Introduction
Although there are multiple reports describing the safe use of short-term parenteral nutrition (PN) in hospitalised women, for example, for hyperemesis gravidarum,1 there are fewer published studies detailing the outcomes and risks of pregnancies in home parenteral nutrition (HPN)-dependent patients. Buchholz and colleagues collated details on 15 pregnancies that had been described in the literature to 20152 and since then, three subsequent case series were published from France,3 the UK,4 Israel and Poland,5 describing a total of 35 pregnancies in 25 women occurring between 1982 and 2016.6 Thus, many healthcare professionals will likely have very limited or no experience of managing pregnancy in HPN-dependent patients.
With an ever-increasing use of HPN worldwide,7 8 it is likely that the occurrence of pregnancy, and considerations relating to fertility and conception in chronic intestinal failure (CIF) will also increase. The current approach of healthcare professionals towards pregnancy in CIF may be influenced by their perception of risk, both to the fetus and to the mother, including the potential for CIF-related complications. There are, however, to date, no published consensus international guidelines on pregnancy considerations in CIF that relate to factors such as conception, PN optimisation before, during and after pregnancy and the risk of HPN-related complications.
Our international survey therefore aimed to evaluate the experience and opinion of multidisciplinary healthcare professionals with specialist interest in CIF, exploring the perceived optimal approach to aspects of pregnancy management, including pre-pregnancy counselling, parenteral support optimisation and HPN-related complication risk.
Methods
An opinion-based survey was produced following detailed multidisciplinary discussion between gastroenterologists (AB, SL, PA, LP), a dietitian (KF) and an obstetrician (LM). The study questionnaire was structured into the following subsections:
Demographics and pregnancy experience.
Opinions and experience of pregnancy.
Optimisation of parenteral support for conception.
Optimisation of parenteral support during pregnancy.
Opinions and experience of CIF and birth.
Optimisation of parenteral support in the postnatal period and for lactation.
An invitation to participate with a weblink to the questionnaire created by the survey software was circulated electronically via newsletters published for members of European Society for Clinical Nutrition and Metabolism. Clinicians and healthcare professionals identified by the study team who have expertise in the management of CIF were also invited to participate in the survey via email. Survey data were collected from November 2021 to February 2022. A web-based survey tool (REDCap) was used to generate the survey questionnaire and collect data.
Data were collected in the form of single or compound answer multiple-choice questions, drop-down menu questions for numerical data, also with open-ended questions for descriptive exploration of clinical practice. The questions were phrased to assess either direct experience or opinion in order to allow for the variation in clinicians’ exposure to pregnancy in patients with CIF. As such, all elements of the survey were available to participants for completion. Survey data were analysed using counts, proportions and where appropriate χ2 testing was applied. Ethics approval was not required since this was a clinician-based opinion and experience survey, with no patient-related clinical data collected, in keeping with the Caldicott principles.
Results
Respondent demographics and pregnancy experience
A total of 70 responses were received from 11 different countries. The mean number of patients cared for with CIF per responder was 114 (range 2–400). Of the 70 respondents, 52/70 (72.4%) were physicians, 9/70 (12.5%) dietitians, with the remaining 9/70 including surgeons, specialist nurse and specialist pharmacists. Only 38/70 (54.2%) of respondents reported having personally cared for at least one pregnant patient with CIF. These respondents described experience of a total of 101 pregnancies in HPN-dependent patients, although the same pregnancy occurrence may, of course, have been described by more than one respondent working within the same CIF service. Seventeen pregnancies (17/101) had an unknown outcome. Of the 84 pregnancies with a known outcome, 15 were reported as having been unsuccessful. When comparing those who had had prior experience of pregnancy in CIF and those that had not there was no significant difference in opinion when considering fertility on women with CIF (p=0.1).
Pregnancy-related discussions and risk perception
Participants were questioned about contraception, fertility and pregnancy risks. The majority (74.2%) stated that they would not routinely discuss contraception with patients and 53 participants (76%) felt that any advice or discussion around conception should be based on an individualised approach. There was no significant difference between those who had and had not managed pregnancy in patients with CIF (p=0.8). None of the respondents who had prior experience suggested they would advise against pregnancy, while only one of those who had no experience expressed this opinion.
Thirty respondents (42.8%) felt they did not have enough experience to comment on the risk of reduced fertility in HPN-dependent patients, while 23/70 (32.8%) felt fertility was determined by the patient’s individualised risk based on underlying CIF disease or clinical condition. Participants were also asked to consider the impact of the underlying disease leading to CIF on the likelihood of conception and successful pregnancy, should the patient wish to or become pregnant. The presence of chronic intestinal pseudo-obstruction (CIPO), followed by radiation enteritis was felt to be the aetiologies which would most reduce the likelihood of conception.
Sixty per cent (42/70) of respondents stated that they did not feel that it was their role, as the specialist in CIF, to discuss the topic of pregnancy with their patients, with fewer than 10% of respondents stating that they routinely discussed pregnancy with patients of childbearing age. When determining risk to the mother and potential fetus, 71% (50/70) and 70% (49/70), respectively, reported that their experience was too limited to provide reliable support or advice to their patients, although 3/70 respondents stated they would actively advise against pregnancy in patients with CIF. Participants were also asked to consider the risk of HPN-related complications in pregnancy. Most felt there was no increased risk of catheter-related blood stream infection (CRBSI), while central venous catheter (CVC)-related thrombosis was deemed to be the most significant HPN-related complication for pregnant women, p=<0.001 (figure 1). This was most noticeable in those who had no prior experience of pregnancy in CIF (p=<0.001). There was no difference in risk perception of CRBSI in those who had or had not had prior experience of pregnancy.
Figure 1.
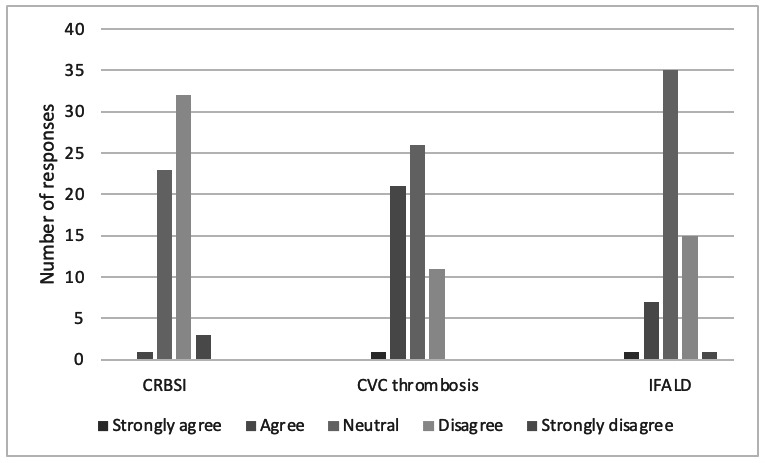
Respondent opinion as to the increased risk of HPN-related complications during pregnancy. CRBSI, catheter-related blood stream infection; CVC, central venous catheter; IFALD, intestinal failure-associated liver disease; HPN, home parenteral nutrition.
Conception and pregnancy optimisation
Participants were asked to give their opinion as to the optimal changes to parenteral support in order to optimise clinical and nutritional status prior to conception (table 1). Most respondents felt that an individualised approach was the most appropriate, with very few suggesting any decrease in the overall support offered. There was no difference for those with or without experience of pregnancy in CIF.
Table 1.
Suggested changes to parenteral support in order to optimise a patient’s clinical condition prior to conception
| Parenteral support changes for conception | |||||
| Increase | Stay the same | Tailor to individual | Decrease | No response | |
| Energy requirement | 10 (14%) | 18 (26%) | 28 (40%) | 0 (0%) | 14 (20%) |
| Lipid content | 3 (4%) | 25 (36%) | 27 (39%) | 1 (1%) | 14 (20%) |
| Protein content | 9 (13%) | 21 (20%) | 25 (36%) | 0 (0%) | 15 (21%) |
| Vitamins/micronutrients | 20 (29%) | 11 (16%) | 25 (36%) | 0 (0%) | 14 (20%) |
When asked about essential fatty acid assessment prior to conception and/or during pregnancy, only 9/70 (12.8%) reported that they would routinely check these levels. Micronutrient and other biochemical assessments considered for optimisation prior to conception are detailed in figure 2; notably, 13/70 (18.5%) stated that they would not assess folate in a patient actively trying to conceive.
Figure 2.
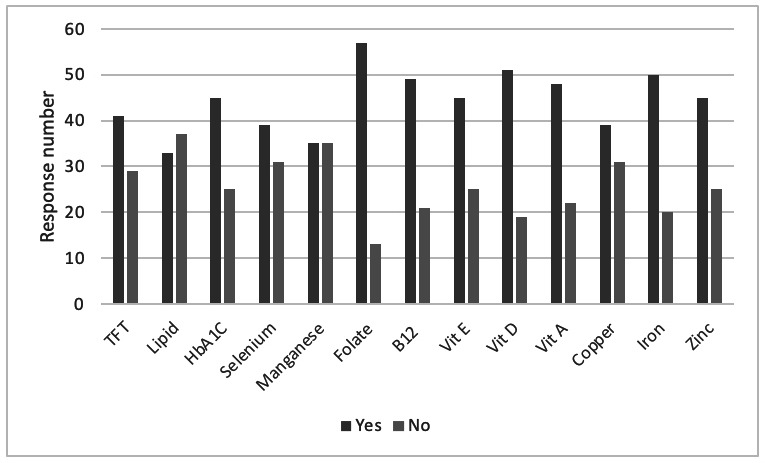
Biochemical and micronutrient parameters that respondents felt should be checked and optimised prior to conception in patients with CIF. CIF, chronic intestinal failure; TFT, thyroid function test.
Participants were also asked about monitoring during pregnancy, and most recommended 4-weekly clinical reviews during all three trimesters. Notably, access to dedicated obstetric care for high-risk pregnancy was reported by 80% of respondents, while three-quarters of participants felt that a specialist IF-pregnancy service improved the care and outcomes for pregnant patients with CIF.
Participants were also asked about nutritional optimisation during pregnancy, with most respondents preferring an individualised approach to PN optimisation during each trimester (table 2). Over 80% of respondents felt that a third generation lipid source, consisting of soya-bean oil, medium-chain triglycerides, olive oil and fish oil was the optimal lipid for patients prior to conception and for use during pregnancy.
Table 2.
Opinion of the best way to optimise parenteral support during the three trimesters of pregnancy
| Parenteral support changes for first trimester (%) | |||||
| Increase | Stay the same | Tailor to individual | Decrease | No response | |
| Energy requirement | 14 | 30 | 25 | 0 | 30 |
| Lipid content | 0 | 35 | 30 | 4 | 30 |
| Protein content | 5 | 37 | 27 | 0 | 3 |
| Vitamins/micronutrients | 25 | 17 | 27 | 0 | 30 |
| Parenteral support changes for second trimester (%) | |||||
| Increase | Stay the same | Tailor to individual | Decrease | No response | |
| Energy requirement | 25 | 11 | 32 | 0 | 30 |
| Lipid content | 14 | 18 | 35 | 1 | 30 |
| Protein content | 14 | 17 | 38 | 0 | 30 |
| Vitamins/micronutrients | 18 | 18 | 31 | 1 | 30 |
| Parenteral support changes for third trimester (%) | |||||
| Increase | Stay the same | Tailor to individual | Decrease | No response | |
| Energy requirement | 38 | 1 | 30 | 0 | 30 |
| Lipid content | 21 | 11 | 35 | 1 | 30 |
| Protein content | 22 | 8 | 38 | 0 | 30 |
| Vitamins/micronutrients | 22 | 12 | 31 | 1 | 31 |
Birth and postnatal management
Participants were asked to consider infant delivery and optimisation of PN during the postnatal period, as well as breast feeding. Eighty-four per cent (59/70) of respondents indicated that the choice of delivery mode should be made on an individualised patient basis, with the remaining 16% indicating they did not have sufficient experience to comment. Participants were asked to indicate which clinical features may prompt a Caesarean section (C-section) as the preferable mode of delivery; the presence of perianal Crohn’s disease was the strongest indicator (figure 3).
Figure 3.
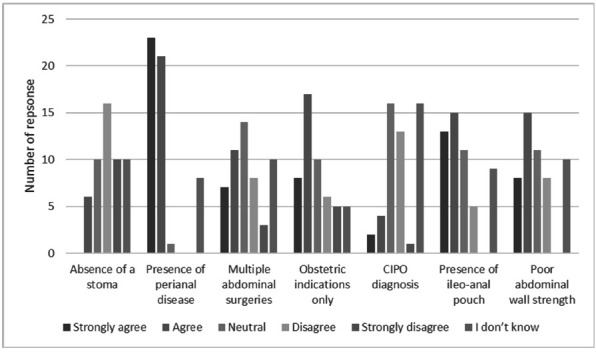
Opinion relating to the indication of C-section as the preferable mode of delivery. CIPO, chronic intestinal pseudo-obstruction.
57/70 (81%) respondents felt breast feeding would be the optimal mode of feeding, with 85% defining maternal choice as the most important factor in decision-making. Participants’ opinions on parenteral support modifications during breast feeding are shown in table 3.
Table 3.
Opinion of the best way to optimise parenteral support during lactation
| Parenteral support changes for lactation (%) | |||||
| Increase | Stay the same | Tailor to individual | Decrease | No response | |
| Energy requirement | 30 | 10 | 22 | 0 | 37 |
| Lipid content | 18 | 11 | 30 | 2 | 37 |
| Protein content | 18 | 12 | 30 | 1 | 37 |
| Vitamins/micronutrients | 8 | 18 | 32 | 2 | 37 |
Discussion
This is the first study to evaluate the experience and opinions of healthcare professionals in managing pregnancy in CIF. Approximately half of all respondents had no experience of managing pregnant patients; over 40% reported insufficient experience to be able to discuss fertility with HPN-dependent people; and the majority of respondents reported insufficient experience and evidence from the literature to provide reliable support or advice regarding the risks of pregnancy in patients with CIF. It would therefore seem that many would benefit from the addition of a dedicated obstetrician to the wider multidisciplinary team (MDT). Indeed, while a minority would actively advise against pregnancy in patients with CIF, most stated that they did not feel it was their role to discuss pregnancy with their patients. Given the—although small numbers of—published reports of safe pregnancy in patients with CIF,2–5 and the likelihood of an increasing number of HPN-dependent patients of childbearing age as the prevalence of CIF continues to increase in many countries,7–9 there is a clear need for a registry to provide further evidence for international guidance on this issue. It must be noted that the scarcity of data and experience is likely to originate from the relative infrequency of pregnancy in CIF. Furthermore, enhanced data capture of both successful and unsuccessful pregnancy occurrences and outcomes within established national7 and international CIF databases10 will undoubtedly add future vital information for clinicians in this important area for patients. The results of this study would appear to support the notion that all members of the MDTs that care for patients with CIF would benefit from further knowledge and expertise when considering fertility and pregnancy.
The results of this paper add some important insight into the current practices of CIF clinicians when caring for patients’ pre-conception, during and following pregnancy. The majority of those able to answer stated that they would tailor HPN requirements to the individual pre-conception and during or following pregnancy. In the largest series of pregnancy in CIF to date from France, patients had an increased PN energy need during pregnancy, especially in the second and third trimesters, and some individuals required more days of PN.3 Similarly, in the UK series, patients also required an increase in energy and nitrogen during pregnancy.4 Notably, neither the French nor the UK series reported using fish-oil based lipids, although the majority of respondents in this survey felt that a third-generation lipid containing fish-oils would be an optimal choice for pregnant patients. Essential fatty acid deficiency possibly associated with the use of such lipids11 is an important consideration in pregnant patients given the potential risk to the fetus12 and clearly more data are required detailing the safety of fish-oil based PN emulsions in CIF. The importance of close blood monitoring of vitamins and trace elements was recognised by respondents of this survey, although only a minority reported checking essential fatty acid levels. In addition, a significant minority of respondents reported not assessing or optimising folate prior to conception, reinforcing the need for enhanced education and guidelines for CIF healthcare professionals in this field.
Maternal complications reported within the French series by Billiauws and colleagues included CRBSI in five patients and cholestasis in one.3 While respondents to this survey did not feel that CRBSI risk was necessarily increased in pregnancy, more reported the occurrence of CVC-related thrombosis to be of concern, which may relate to the prothrombotic risk of pregnancy.13 Most respondents did not feel that intestinal failure-associated liver disease risk was increased in pregnancy. However, it is clear that more data, ideally collected prospectively from multiple CIF centres, are required on the risk of CIF complications in pregnancy, and the role and safety of strategies such as anti-microbial locks to mitigate these risks. Other risks to the mother that need to be considered include exacerbation of the underlying disease leading to CIF; while there are established guidelines on the management of conditions such as Crohn’s disease in pregnancy that can readily be extrapolated to the CIF population,14 Billiauws and colleagues reported concerning complications in two mothers with CIPO and highlighted that this group of HPN-dependent patients required close follow-up.3 The majority of respondents to our survey advocated at least 4-weekly clinical reviews for pregnant patients, with most having access to high-risk obstetric care. Moreover, over 80% of respondents felt that the choice of delivery mode should be individualised to the patient, with perianal Crohn’s being the strongest indication for C-section. Clearly, close collaboration between the obstetric and CIF teams is vital during and following pregnancy and underscores the importance of access to appropriate high-risk obstetric services.
Due to the retrospective nature of the data collected, some participants were unable to recall the outcomes for many of the defined pregnancies. However, 18% of the recorded pregnancies were defined as unsuccessful. In the UK, 1 in 5 pregnancies ends in miscarriage and although the questionnaire did not explore the specific details of successful and unsuccessful pregnancies. It would appear that reported outcomes here are in line with the general population.15
Conclusion
This study highlights the need for multicentre data collection, potentially via an international registry, further education and consensus guidelines to better equip CIF clinicians and patients in their decision-making prior to, during and following pregnancy. As the number of HPN-dependent patients of childbearing age will increase internationally, the ultimate goal is that CIF healthcare professionals and patients feel confident in discussing pregnancy-related issues so that informed choices can be made.
flgastro-2023-102384supp001.pdf (19.6KB, pdf)
flgastro-2023-102384supp002.pdf (38.8KB, pdf)
flgastro-2023-102384supp003.pdf (20KB, pdf)
flgastro-2023-102384supp004.pdf (31KB, pdf)
flgastro-2023-102384supp005.pdf (19.5KB, pdf)
flgastro-2023-102384supp006.pdf (31.9KB, pdf)
flgastro-2023-102384supp007.pdf (39.7KB, pdf)
Footnotes
Twitter: @drthomasconley
Contributors: AB, SL, PA, KF, LM contributed to conceptualisation. AB, SL, PA, KF contributed to methodology. AB, SL contributed to software. AB and SL contributed to formal analysis. AB contributed to data curation. AB, LP, SL, PA contributed to writing–original draft preparation. AB, PA, TEC, KF, LM, FB, CC, PJ, FJ, GL, MM, KS, AVG, GW, LP, SL contributed to writing–review & editing. SL contributed to supervision. AB contributed to project administration and is the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Fejzo MS, Ingles SA, Wilson M, et al. High prevalence of severe nausea and vomiting of pregnancy and hyperemesis gravidarum among relatives of affected individuals. Eur J Obstet Gynecol Reprod Biol 2008;141:13–7. 10.1016/j.ejogrb.2008.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buchholz BM, Rüland A, Kiefer N, et al. Conception, pregnancy, and lactation despite chronic intestinal failure requiring home parenteral nutrition. Nutr Clin Pract 2015;30:807–14. 10.1177/0884533615574003 [DOI] [PubMed] [Google Scholar]
- 3. Billiauws L, Armengol Debeir L, Poullenot F, et al. Pregnancy is possible on long-term home parenteral nutrition in patients with chronic intestinal failure: results of a long term retrospective observational study. Clin Nutr 2017;36:1165–9.:S0261-5614(16)30204-7. 10.1016/j.clnu.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 4. Bond A, Vasant DH, Gashau W, et al. Managing successful pregnancies in patients with chronic intestinal failure on home parenteral nutrition: experience from a UK national intestinal failure unit. J Gastrointestin Liver Dis 2017;26:375–9. 10.15403/jgld.2014.1121.264.ukn [DOI] [PubMed] [Google Scholar]
- 5. Theilla M, Ławiński M, Cohen J, et al. Safety of home parenteral nutrition during pregnancy. Clin Nutr 2017;36:288–92.:S0261-5614(15)00338-6. 10.1016/j.clnu.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 6. Moreno JM, Gomis P. Pregnancy in a patient with chronic intestinal failure on long-term parenteral nutrition. Clin Nutr 2002;21:438–40. 10.1054/clnu.2002.0577 [DOI] [PubMed] [Google Scholar]
- 7. Smith T, Naghibi M. British artificial nutrition survey (BANS) report 2016. In: Artificial Nutrition Support in the UK 2005-2015. Adult Home Parenteral Nutrition & Home Intravenous Fluids. 2016: 1–27. [Google Scholar]
- 8. Dibb M, et al. Survival and nutritional dependence on home parenteral nutrition: three decades of experience from a single referral centre. Clinical Nutrition 2016;16:S0261–5614. [DOI] [PubMed] [Google Scholar]
- 9. Brandt CF, Tribler S, Hvistendahl M, et al. Single-Center, adult chronic intestinal failure cohort analyzed according to the ESPEN-endorsed recommendations, definitions, and classifications. JPEN J Parenter Enteral Nutr 2017;41:566–74. 10.1177/0148607115612040 [DOI] [PubMed] [Google Scholar]
- 10. Pironi L, Steiger E, Joly F, et al. Intravenous supplementation type and volume are associated with 1-year outcome and major complications in patients with chronic intestinal failure. Gut 2020;69:1787–95. 10.1136/gutjnl-2018-318172 [DOI] [PubMed] [Google Scholar]
- 11. Memon N, Hussein K, Hegyi T, et al. Essential fatty acid deficiency with smoflipid reduction in an infant with intestinal failure-associated liver disease. JPEN J Parenter Enteral Nutr 2019;43:438–41. 10.1002/jpen.1432 [DOI] [PubMed] [Google Scholar]
- 12. Skoracka K, Ratajczak AE, Rychter AM, et al. Female fertility and the nutritional approach: the most essential aspects. Adv Nutr 2021;12:2372–86. 10.1093/advances/nmab068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Konkle BA. DIAGNOSIS AND MANAGEMENT OF THROMBOSIS IN PREGNANCY: DIAGNOSIS AND MANAGEMENT OF THROMBOSIS IN PREGNANCY. Birth Defect Res C 2015;105:185–9. [DOI] [PubMed] [Google Scholar]
- 14. van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis 2015;9:107–24. 10.1093/ecco-jcc/jju006 [DOI] [PubMed] [Google Scholar]
- 15. Miscarriage Association . Background information: miscarriage. 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
flgastro-2023-102384supp001.pdf (19.6KB, pdf)
flgastro-2023-102384supp002.pdf (38.8KB, pdf)
flgastro-2023-102384supp003.pdf (20KB, pdf)
flgastro-2023-102384supp004.pdf (31KB, pdf)
flgastro-2023-102384supp005.pdf (19.5KB, pdf)
flgastro-2023-102384supp006.pdf (31.9KB, pdf)
flgastro-2023-102384supp007.pdf (39.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request.


