Abstract
Objectives:
To establish consensus practices among a panel of national experts for the discharge of premature infants with bronchopulmonary dysplasia (BPD) from the hospital to home.
Study design:
We conducted a Delphi study that included US neonatologists and pediatric pulmonologists from the BPD Collaborative to establish consensus practices, defined as recommendations with at least 80% agreement, for infants with BPD being discharged from the hospital. Specifically, we evaluated recommendations for diagnostics to be completed around discharge, follow-up respiratory care, and family education.
Results:
Thirty-one expert participants completed three rounds of surveys, with a 92/93 (99%) response rate. Consensus was established that infants with moderate-severe BPD (those who remain on respiratory support at 36 weeks) and those discharged on oxygen should be targeted for in-person pulmonary follow-up within 1 month of hospital discharge. Specialized neonatal follow-up is an alternative for infants with mild BPD. Those with moderate or severe BPD should have an echocardiogram performed after 36 weeks to screen for pulmonary hypertension. Infants with BPD warrant additional evaluations if they have growth restriction or poor growth, pulmonary hypertension, tachypnea, and if they are discharged home on oxygen, diuretics, or non-oral feeds.
Conclusion:
This Delphi survey establishes expert consensus around best practices for follow-up respiratory management and routine evaluations for infants with BPD surrounding neonatal discharge. Areas of disagreement where consensus was not established are discussed.
Keywords: Delphi survey, chronic lung disease of prematurity, neonatal transitions of care
Introduction
Premature infants with bronchopulmonary dysplasia (BPD) have complex care needs surrounding discharge from the Neonatal Intensive Care Unit (NICU). BPD affects up to 40% of former preterm infants born at <28 weeks gestation, with up to 50,000 new cases of BPD in the United States every year.[1–3] These infants may require ongoing respiratory care, management of medications and home respiratory support, management of pulmonary hypertension, monitoring of growth and nutrition, and developmental follow-up.[4–6] Discharge from NICU to home is a high-risk transition of care, with up to 50% of infants with BPD requiring rehospitalization in the first year alone.[7] Coordinated follow-up programs may help improve outcomes and reduce rates of hospitalization in this population.[8] There are diverse follow-up and management strategies employed by outpatient BPD clinics in the United States.[9]
Though recently published guidelines establish recommendations for care of children with post-prematurity respiratory disease, these do not address which infants discharging from the NICU should be targeted for specialized follow-up for their respiratory disease, which evaluations should be routinely performed around discharge, and what family education to provide to optimize successful discharge.[10,11] Additionally, the role of telemedicine for follow-up in this population is unclear, despite an increased reliance on its use during the SARS-CoV-2 pandemic.[12,13] We conducted a Delphi study among neonatologists and pulmonologists from the BPD Collaborative to determine consensus practices in the discharge of infants <32 weeks gestational age (GA) from the NICU with BPD. We hypothesized that we would be able to establish “best practices” for identifying BPD infants who require specialized follow-up and additional evaluations around NICU discharge.
Methods
Delphi panel participants
The Delphi method aims to identify areas of consensus practices among a group of expert participants using iterative surveys.[14] For this study, consensus was defined a priori as ≥ 80% agreement among participants. Where consensus could not be attained an explanation is provided as to the sources of disagreement. We recruited members of the BPD collaborative, a consortium of 25 pediatric centers with expertise in the treatment of BPD, to serve as participants on the BPD Collaborative Expert Panel on Discharge Practices. A purposeful sample was designed to include both neonatologists and pediatric pulmonologists across multiple regions of the United States. Eligible participants were informed of the study requirement, which included participation in all surveys distributed to attain a 100% response rate and to maintain confidentiality around their responses.
The study was granted a waiver of consent from the institutional review board at the coordinating center. A participant agreement form was included with the first questionnaire.
Questionnaires
Iterative questionnaires were administered recursively. Each round was designed based on previous responses until consensus was achieved among participants or reasons for disagreement were understood. Three rounds of surveys were required. The initial questionnaire was tested for content and modified based on responses by three expert non-participants. Questionnaires included four case vignettes and one question about family education and counseling. The four vignettes described preterm neonates <32 weeks GA nearing discharge with mild, moderate, and severe BPD, as well as one with severe BPD requiring supplemental oxygen at discharge. BPD severity was defined based on the 2001 National Institute of Child Health and Human Development/National Heart Lung and Blood Institute consensus definition, using respiratory support at 36 weeks post-menstrual age (PMA).[15] In the each primary vignette presentation no other diagnoses were described other than BPD, and it was explicitly stated that no medications were being used at discharge to treat their BPD.
Survey methodology is outlined in Figure 1. In round one, each participant was asked open-ended questions about the patient vignettes regarding need for specialty follow-up specific to their respiratory condition, and if so, the ideal time frame and format (in person and/or telehealth). Participants were asked about other routine referrals or testing they would recommend for these infants before or shortly after discharge. In addition, we asked open-ended questions, with examples, about how responses might change based on the following: different gestational ages, growth issues including history of intrauterine growth restriction (IUGR) or poor postnatal growth, comorbid diagnoses or symptoms (including persistent tachypnea, a history of prolonged spells, ongoing or resolved pulmonary hypertension, a patent ductus arteriosus, atrial septal defect, nasogastric tube at discharge, resolved pneumonia, or resolved pneumothorax), and medication use (including diuretics, systemic steroids, and inhaled respiratory medications). Finally, we asked what education or counseling the participant would recommend providing to families prior to NICU discharge. For each question, we encouraged participants to explain the rationale behind their responses.
Figure 1. Description of Delphi Survey Rounds.
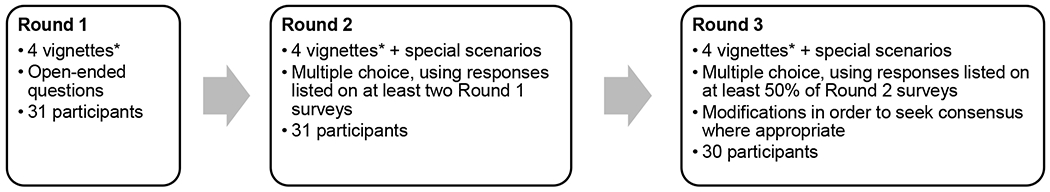
*The four vignettes included in the survey were preterm infants <32 weeks gestation with mild, moderate, and severe BPD by 2001 NHLBI criteria, plus one with severe BPD discharge on home oxygen.
The second round summarized the open-ended responses in round one, highlighting modal responses to each question, areas of consensus, and all responses listed by at least two participants. For each vignette, we asked participants to choose responses with which they agreed, and again encouraged open-ended responses to explain rationale for agreement or disagreement with the listed responses.
In the third and final round, we included responses that garnered agreement of at least 50% of participants from the round two survey but had not already attained consensus. Where appropriate, responses and questions were combined to create consensus recommendations. Participants were asked to agree or disagree with recommendations, and again, encouraged to describe the rationale to their decision.
Data collection
Surveys were completed using REDCap over a 2-3 week time span for each round.[16,17] Individual follow-up was used to request response when participants were late. Participant responses remained anonymous to all except the study coordinator, who had access to names if clarifications were needed.
The first two rounds, which each contained open-ended questions, were scored by two study team members (JL and CA). Any response for which there was > 10% disagreement in labeling by scorers was further reviewed. There was 85% initial agreement among scorers.
Results
Thirty-one experts from 19 centers from the BPD collaborative participated in the Delphi survey, including 14 neonatologists and 18 pediatric pulmonologists (one participant practices in both specialties) (Table 1; online). Experts represented areas across the US, with regional locations of home institutions including: Midwest 5, South 7, Northeast 8, West 11. Participants responded to 92/93 (99%) of surveys; 100% (31/31) for rounds one and two, and 97% (30/31) for round three.
Table 1.
Sampling table of expert participants
| Region of United States | |||||
|---|---|---|---|---|---|
| Northeast | South | West | Midwest | ||
| Specialization | Neonatologist | 5* | 3 | 3 | 3 |
| Pediatric Pulmonologist | 4* | 4 | 8 | 2 | |
Note that one participant practices as a neonatologist and pulmonologist
Areas of Consensus and Disagreement
Expert questionnaire responses by case vignette and survey round are presented in Table 2, including the degree of consensus achieved. Educational recommendations are presented in Table 3. Consensus recommendations are summarized in Table 4.
Table 2.
Responses to case vignettes by survey round
| Discharge recommendation | Mild BPD | Moderate BPD | Severe BPD | BPD Discharge on Oxygen | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Round 1 n=31 |
Round 2 n=31 |
Round 3 n=30 |
Round 1 n=31 |
Round 2 n=31 |
Round 3 n=30 |
Round 1 n=31 |
Round 2 n=31 |
Round 3 n=30 |
Round 1 n=31 |
Round 2 n=31 |
Round 3 n=30 |
|
| Specialty Follow-up | ||||||||||||
| Pulmonary follow-up | 22 (71%) | 22 (71%) | 28 (93%) * | 23 (74%) | 26 (83%) | 26 (84%) | 28 (90%) | 27 (87%) | 28 (90%) | |||
| Neonatal follow-up | 8 (26%) | 10 (32%) | 7 (23%) | 7 (23%) | 6 (19%) | 5 (16%) | 6 (19%) | 4 (31%) | ||||
| No specialty follow-up | 7 (22%) | 5 (16%) | 4 (13%) | 1 (3%) | 1 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| Timing of Initial Follow-up | ||||||||||||
| 1-2 months | 17/21 (81%) | 25/26 (96%) | 19/23 (83%) | 29/30 (97%) | 23/26 (88%) | 29 (94%) | 27/27 (100%) | 31 (100%) | ||||
| 3-6 months | 3/21 (14%) | 1/26 (4%) | 4/23 (17%) | 1/30 (3%) | 3/26 (12%) | 2 (6%) | 0/27 (0%) | 0 (0%) | ||||
| Location of Visit (initial) | ||||||||||||
| In-person | 19/21 (90%) | 25/26 (96%) | 19/23 (83%) | 29/30 (97%) | 21/24 (88%) | 30 (97%) | 22/23 (96%) | 29 (94%) | ||||
| Virtual/Telehealth | 8/21 (38%) | 5/26 (19%) | 7/23 (30%) | 3/30 (10%) | 9/24 (38%) | 3 (10%) | 2 (9%) | 0 (0%) | ||||
| Seasonally dependent | 1/21 (5%) | 1/26 (4%) | 1/23 (4%) | 1/30 (3%) | 1/24 (4%) | 1 (3%) | 1 (4%) | 2 (6%) | ||||
| Routine referrals | ||||||||||||
| Development | 29 (94%) | 26 (84%) | 26 (84%) | 25 (81%) | ||||||||
| Nutrition | 8 (26%) | 15 (48%) | 11 (35%) | 15 (48%) | 11 (35%) | 20 (65%) | 11 (35%) | 23 (74%) | ||||
| Cardiology | 1 (3%) | 2 (6%) | 2 (6%) | 2 (6%) | 4 (12%) | 7 (23%) | 5 (16%) | |||||
| Routine testing | ||||||||||||
| Echocardiogram | 14 (45%) | 22 (71%) | 14 (47%) | 21 (68%) | 27 (87%) | 25 (81%) | 27 (87%) | |||||
| Chest radiograph | 10 (32%) | 16 (59%) | 12 (41%) | 9 (29%) | 19 (61%) | 18 (60%) | 11 (35%) | 19 (61%) | 23 (77%) | 16 (52%) | 23 (74%) | 25 (83%) |
| Blood gas or chemistry | 11 (35%) | 12 (39%) | 11 (35%) | 13 (42%) | 12 (39%) | 13 (42%) | 16 (52%) | 19 (61%) | 22 (73%) | |||
| Feeding evaluation | 7 (23%) | 13 (42%) | 5 (16%) | 12 (39%) | 3 (10%) | 11 (35%) | 5 (16%) | 14 (45%) | ||||
| Oximetry or sleep study | 3 (10%) | 5 (16%) | 4 (13%) | 8 (26%) | 5 (16%) | 10 (32%) | 10 (32%) | 11 (35%) | ||||
| Vit D, Calcium Level | 4 (13%) | 5 (16%) | 3 (10%) | 4 (13%) | 3 (10%) | 5 (16%) | 4 (13%) | 7 (23%) | ||||
 > 80% agreement
> 80% agreement
 70-80% agreement
70-80% agreement
 60-70% agreement
60-70% agreement
 50-60% agreement
50-60% agreement
 < 50% agreement
< 50% agreement
Indicates agreement around consensus statement modified from round 2 responses (see text).
Responses may total >100% as multiple-choice elections were permitted for certain questions. Total assumed to be all participants, other than where explicitly listed (due to branching responses). Only responses with at least 2 respondents in round 1 are listed. BPD definitions and severity among infants <32 weeks gestation based on 2001 NIH consensus criteria
Table 3.
Counseling and education prior to discharge
| Topic | Agreement, no. (%) n= 31 |
|---|---|
| Topics with consensus | |
| Cardiopulmonary resuscitation training | 25 (81%) * |
| Avoiding infectious exposures | 25 (81%) * |
| Observing/monitoring work of breathing | 80 (90%) |
| Importance of palivizumab prophylaxis (if applicable) and influenza vaccination | 27 (87%) |
| Equipment and medication teaching (if applicable) | 31 (100%) |
| Safe sleep | 29 (94%) |
| Symptoms of feeding difficulties and aspiration | 25 (81%) |
| Avoidance of secondhand smoke exposure | 30 (97%) |
| Hand hygiene | 29 (94%) |
| Topics not reaching consensus | |
| Long-term respiratory outcomes of prematurity | 23 (74%) |
| Consultation prior to air travel | 14 (45%) |
 ≥ 80% agreement
≥ 80% agreement
 70-80% agreement
70-80% agreement
 60-70% agreement
60-70% agreement
 50-60% agreement
50-60% agreement
 < 50% agreement
< 50% agreement
Consensus was achieved in round 1; otherwise agreement numbers and percent listed are from round 2 of the Delphi survey.
Table 4.
Summary of Consensus Discharge Practices for BPD Infants <32 Weeks Gestation
| Mild BPD |
| 1. Preterm infants with mild BPD should see neonatal follow-up with at least a one-time pulmonary consultation if resources allow, and more so if any symptoms/complications develop. This visit is preferred within 1-2 months of discharge, in-person if able. 2. They should also have specialized neonatal developmental follow-up. |
| Moderate BPD |
| 1. Preterm infants with moderate BPD should have pulmonary follow-up after NICU discharge if available. This visit is preferred within 1-2 months of discharge, in-person if able. 2. They should also have specialized neonatal developmental follow-up. 3. A routine echocardiogram should be obtained after 36 weeks post menstrual age to screen for pulmonary hypertension. |
| Severe BPD |
| 1. Preterm infants with severe BPD should have pulmonary follow-up after NICU discharge. This visit is preferred within 1-2 months of discharge, in-person if able. 2. They should also have specialized neonatal developmental follow-up. 3. A routine echocardiogram should be obtained after 36 weeks post menstrual age to screen for pulmonary hypertension. |
|
Severe BPD discharged on home oxygen 1. Preterm infants with BPD discharged on oxygen should have pulmonary follow-up after NICU discharge. This visit is preferred within 1-2 months of discharge (within 2 weeks may be preferred), in-person if able (although telehealth visit may be an acceptable alternative if the in-person assessment is delayed). 2. They should also have specialized neonatal developmental follow-up. 3. A routine echocardiogram should be obtained after 36 weeks post menstrual age to screen for pulmonary hypertension. 4. A baseline chest radiograph should be obtained near discharge or soon after discharge. |
| Special scenarios for BPD infants |
| 1. Intrauterine growth restricted infants should have a routine echocardiogram performed after 36 weeks post menstrual age to screen for pulmonary hypertension. 2. Poor postnatal growth infants should have: a. A routine echocardiogram performed after 36 weeks post menstrual age to screen for pulmonary hypertension. b. A formal feeding evaluation. c. Nutrition and/or Gastroenterology follow-up in addition to above follow-up. 3. Discharged on diuretics or with history of prolonged (>1 week) diuretic use should have a chemistry obtained prior to discharge. 4. Discharged on non-oral feeds (for example, nasograstic or gastric) should have a formal feeding evaluation, if safely able. 5. Discharged with ongoing tachypnea should have sooner follow-up for respiratory care, as well as a chest radiograph and a formal feeding evaluation performed around discharge. 6. Diagnosed with ongoing pulmonary hypertension should have, in addition to echocardiography and specialized follow-up, a chest radiograph and sooner follow-up for respiratory care. |
|
Teaching 1. For infants with BPD, the following topics should be covered around neonatal discharge: a. CPR training b. Avoiding infectious exposures c. Observing and monitoring for work of breathing d. Importance of RSV prophylaxis (if applicable) and influenza vaccination e. Avoiding group childcare if able f. Equipment and medication teaching, as appropriate g. Safe sleep h. Monitoring for signs of feeding difficulties and aspiration i. Avoidance of secondhand smoke exposure j. Hand Hygiene |
Bronchopulmonary Dysplasia (BPD) definitions and severity based on 2001 NIH consensus criteria. Consensus was defined as agreement >80%.
Consensus by BPD severity
Consensus was gained that preterm infants with moderate and severe BPD should have a pulmonary consultation after NICU discharge if available (Table 2). This visit is preferred within 1-2 months of discharge, in-person if able. Experts acknowledged that where pediatric pulmonary resources are scarce neonatal follow-up may serve the role of respiratory screening and assessment, but also noting these infants are at increased risk for early and late pulmonary complications.[18] Additionally, experts recognized that in certain areas of the country BPD-focused clinics are staffed by neonatologists. In-person assessments were preferred to obtain an accurate weight and other growth measurements, and to obtain vital signs including oxygen saturation. It was suggested that telehealth may be a more acceptable alternative if those data were available by remote monitoring.
For mild BPD, there were multiple responses in round 2 that individually failed to gain consensus. Consensus was ultimately gained these infants should see neonatal follow-up with a one-time pulmonary consultation if resources allow, and more so if any symptoms/complications develop. Experts reflected that universal pulmonary follow-up may be difficult in certain areas due to limited resources, though at minimum, a respiratory assessment by a neonatal follow-up is specialist is recommended because even mild BPD is associated with early respiratory complications and later abnormalities in pulmonary function.[19–21]
For infants with moderate or severe BPD, consensus was gained that an echocardiogram should be obtained after 36 weeks PMA, prior to discharge. For severe BPD, there was near consensus about obtaining a routine chest radiograph prior to or soon after discharge, with some experts commenting that assessments should be symptom-based rather than based on BPD severity.
Discharge on home oxygen
There was agreement that infants with BPD discharged on supplemental oxygen should have pulmonary follow-up within 1-2 months (some suggested within 2 weeks) and in-person if able (although telehealth visit may be an acceptable alternative if in-person assessment is delayed). Participants cited the importance of monitoring weight gain and oxygen saturation, ascertaining comfort with home oxygen, assessing ability to wean, and troubleshooting equipment issues. Consensus was also gained on obtaining an echocardiogram after 36 weeks PMA, prior to discharge, and obtaining a routine chest radiograph prior to or soon after discharge. There was not consensus (22/30, 73%) on whether a blood gas or chemistry (for bicarbonate) should be obtained; some suggested end-tidal carbon dioxide as a non-invasive alternative.
Education and Counseling
Consensus was reached on several counseling topics with families prior to discharge (Table 3).
Special Scenarios
In the first (open-ended) round, the following diagnoses and situations for infants with BPD were included by participants: IUGR, poor postnatal growth, diuretic use, systemic corticosteroid use, bronchodilator use, history of prolonged spells, discharge with non-oral feeds, persistent tachypnea, and pulmonary hypertension (Table 5; online). Consensus was reached on:
Table 5:
Delphi responses for rounds 2 and 3 for special scenarios
| Special scenario Discharge Recommendation |
Round 2 n=31 |
Round 3 n= 30 |
|---|---|---|
| Intrauterine growth restriction | ||
| Echocardiogram | 22 (71%) | 28 (93%) |
| Poor growth | ||
| Nutrition/gastroenterology referral | 29 (94%) | |
| Feeding and/or swallow evaluation | 25 (81%) | |
| Echocardiogram after 36 weeks PMA | 18 (58%) | 28 (93%) |
| Expedited follow-up within 1 month of discharge | 18 (58%) | 24 (80%) |
| Overnight oximetry or sleep study | 15 (48%) | |
| Chest radiograph | 13 (42%) | |
| Vitamin D, Calcium Level | 10 (32%) | |
| Chemistry | 9 (29%) | |
| Non-oral feeds at discharge (e.g. nasogastric or gastric feeds) | ||
| Feeding evaluation | 25 (81%) | |
| Expedited follow-up within 1 month of discharge | 11 (35%) | |
| ENT and/or aerodigestive referral | 9 (29%) | |
| Chest radiograph | 6 (19%) | |
| Overnight oximetry and/or sleep study | 3 (10%) | |
| Persistent tachypnea | ||
| Expedited follow-up within 1 month of discharge | 26 (84%) | |
| Chest radiograph | 22 (71%) | 26 (87%) |
| Feeding and/or swallow evaluation | 19 (61%) | 26 (87%) |
| Overnight oximetry and/or sleep study | 15 (48%) | |
| ENT and/or aerodigestive referral | 11 (35%) | |
| Diuretic use at discharge | ||
| Chemistry | 25 (81%) | |
| Expedited follow-up within 1 month of discharge | 19 (61%) | 24 (80%) |
| Echocardiogram after 36 weeks PMA | 10 (32%) | |
| Chest radiograph | 10 (32%) | |
| Blood gas | 9 (29%) | |
| Renal Ultrasound | 7 (23%) | |
| History of significant diuretic use (>1 week) | ||
| Chemistry | 17 (55%) | 25 (83%) |
| Renal Ultrasound | 10 (32%) | |
| Blood gas | 9 (29%) | |
| Expedited follow-up within 1 month of discharge | 9 (29%) | |
| Chest radiograph | 8 (26%) | |
| Echocardiogram after 36 weeks PMA | 7 (23%) | |
| Significant corticosteroid exposure (>1 course or prolonged taper) | ||
| Cortisol | 16 (52%) | 13 (43%) |
| Expedited follow-up within 1 month of discharge | 11 (35%) | |
| Chest radiograph | 11 (35%) | |
| Echocardiogram after 36 weeks PMA | 10 (32%) | |
| Blood gas | 8 (26%) | |
| Chemistry | 6 (19%) | |
| Pulmonary hypertension ongoing | ||
| Expedited follow-up within 1 month of discharge | 23 (74%) | 25 (83%) |
| Chest radiograph | 17 (55%) | 28 (93%) |
| Overnight oximetry and/or sleep study | 20 (65%) | 23 (77%) |
| Feeding and/or swallow evaluation | 14 (45%) | |
| ENT and/or aerodigestive referral | 2 (6%) | |
| Inhaled bronchodilators or steroid | ||
| Expedited follow-up within 1 month of discharge | 18 (58%) | 20 (67%) |
| Chest radiograph | 13 (42%) | |
| Echocardiogram after 36 weeks PMA | 9 (29%) | |
| Blood gas | 8 (25%) | |
| Chemistry | 5 (16%) | |
| Cortisol | 3 (10%) | |
| Prolonged history of spells | ||
| Expedited follow-up within 1 month of discharge | 17 (55%) | 23 (77%) |
| Feeding and/or swallow evaluation | 14 (45%) | |
| Chest radiograph | 12 (39%) | |
| Overnight oximetry and/or sleep study | 12 (39%) | |
| Otorhinolaryngology and/or aerodigestive referral | 8 (26%) |
 ≥ 80% agreement
≥ 80% agreement
 70-80% agreement
70-80% agreement
 60-70% agreement
60-70% agreement
 50-60% agreement
50-60% agreement
 < 50% agreement
< 50% agreement
Responses may total > 100% as multiple-choice elections were permitted for certain questions. Total assumed to be all participants, other than where explicitly listed (due to branching responses). Only responses with at least 2 respondents in round 2 are listed. Expedited follow-up indicates priority for follow-up under 1 month of NICU discharge. BPD definitions and severity among infants <32 weeks gestation based on 2001 NIH consensus criteria. Note round 1 responses were open-ended to describe special scenarios and thus responses were not included in this table.
For BPD infants with IUGR, obtaining an echocardiogram after 36 weeks PMA to screen for pulmonary hypertension, due to increased risk
For BPD infants with poor postnatal growth, obtaining nutrition and/or gastroenterology follow-up, a feeding evaluation and/or swallow study, and an echocardiogram
For BPD infants requiring non-oral feeds at discharge (for example, nasogastric or gastric), obtaining a feeding evaluation and/or swallow study, if safely able to participate.
For BPD infants with persistent tachypnea near discharge, obtaining a chest radiograph and a feeding evaluation.
For BPD infants on diuretics at discharge or with a history of greater than 1 week of diuretic use, obtaining a blood chemistry prior to discharge.
For BPD infants with pulmonary hypertension, obtaining a chest radiograph, along with follow-up echocardiograms as standard of care for this condition. Many would obtain overnight oximetry or a sleep study, but there was not consensus on this.
Infants with poor postnatal growth, persistent tachypnea, pulmonary hypertension, and those on diuretics should be prioritized within one month for follow-up, per consensus.
There was no consensus if infants with a history of significant systemic corticosteroid exposure, defined as >1 course or prolonged taper, should have a cortisol level obtained. Respondents commented on a lack of evidence supporting the utility of cortisol levels in preterm infants. There was also no consensus around practices for BPD infants discharged on inhaled respiratory medications or with prolonged episodes of apnea, desaturation, or bradycardia.
Areas of Agreement by Specialty
Overall, responses were similar by specialty area. The only response that was significantly different between pulmonologists and neonatologists was oximetry for BPD infants with pulmonary hypertension; a greater proportion of pulmonologists (15/16, 94%) than neonatologists (8/14, 57%) agreed that it should be routinely performed prior to discharge.
Discussion
This Delphi study provides the first set of consensus practices for providers around NICU discharge practices for infants <32 weeks GA with BPD. The panel of 31 pulmonologists and neonatologists from 19 BPD Collaborative centers across the US identified areas of consensus on studies or evaluations to obtain prior to discharge, infants to prioritize for pulmonary follow-up, timing of follow-up, which comorbid diagnoses or circumstances should be considered, and areas of educational emphasis.
Expert panelists were in consensus that infants with moderate-severe BPD, who remain on respiratory support at 36 weeks PMA, and those discharged on oxygen should be prioritized for pediatric pulmonary follow-up, which should be within one month of neonatal discharge and in-person to allow for collection of vital signs, weight, and measurements. Infants with mild BPD should be monitored for pulmonary complications with pulmonary consultation as resources allow, as they remain at risk of symptoms, medication use, rehospitalization, and lower lung function in childhood.[18,22,23]
In addition, there was consensus that infants with moderate or severe BPD have routine screening for pulmonary hypertension by echocardiogram, which is supported by existing American Heart Association and American Thoracic Society guidelines, though there is some variation in the applicability of this practice.[24,25] A routine chest radiograph near discharge reached near consensus in infants with severe BPD and reached consensus for infants on home oxygen. Other routine tests were suggested by some participants but did not reach consensus, such as blood gas or bicarbonate (on a serum chemistry panel), but could be considered on an individualized basis.
Several comorbidities and specific scenarios warranted additional evaluations by consensus, including IUGR infants, infants with poor growth, infants with pulmonary hypertension, infants requiring non-oral feeds at discharge, infants on diuretics or with significant (>1 week) diuretic exposure, and infants with tachypnea.[26–28] Notably there was no clear consensus on whether infants with a history of prolonged corticosteroid exposure should have further evaluation for adrenal insufficiency, with many citing a lack of consistent data to provide normative cortisol levels in this population.[29]
Overall, this survey provides a set of best practices regarding the discharge management of infants with BPD based on expert consensus, rather than empirical evidence. The authors believe that in the absence of such evidence, these consensus recommendations are a useful alternative to guide practice. Efficacy will need to be tested in implementation studies. Additionally, topics without consensus highlight areas where further research is needed.
This survey complements recent society guidelines from the American Thoracic Society and European Respiratory Society regarding the care of infants and children with BPD and post-prematurity respiratory disease, which address medication use and the role of diagnostic testing in the outpatient setting.[10,11] These guidelines do not comment on the need for specialized follow-up for these infants, and do not formally distinguish disease severity or degree of prematurity. The results of this Delphi survey consensus portray how expert providers use disease severity to triage infants <32 weeks GA at highest priority for specialist follow-up and additional diagnostic testing around NICU discharge.
The Expert Panel on Discharge Practices queried in this study included geographically diverse neonatologists and pulmonologists recruited from the BPD Collaborative, an organization comprised of pediatric tertiary and quaternary care institutions with multidisciplinary teams dedicated to optimizing outcomes of infants and children with severe BPD. Neonatologist and pulmonologist recommendations in this study were similar. Other strengths include the high response rate (97%), anonymous responses that were given equal weight in analysis, and identification of rationale where there were areas of disagreement.
All panel participants, while considered experts, also practice in academic centers providing this subspecialized care; there may be resource limitations in implementing these consensus findings in diverse practice settings. Other limitations include that we asked only about infants <32 weeks GA with BPD based on the 2001 consensus definition. Recommendations for infants with BPD ≥32 weeks or alternative BPD definitions were not queried in this survey.[30,31] However, the updated definition published by the Jensen et al. with the Neonatal Research Network can be applied to the case scenarios described in the survey, notably: the mild BPD case is equivalent to no BPD (Jensen), the moderate BPD case is equivalent to Grade 1 BPD, severe BPD case (in this scenario, on non-invasive support) is equivalent to Grade 2 BPD. Grade 3 BPD was not included in the survey, as there are likely distinct considerations for infants’ still mechanically ventilated at 36 weeks’ PMA.
In summary, despite a lack of evidence to guide discharge of infants with BPD from the NICU, an expert panel of specialists reached consensus on a wide range of practices around discharge, including how to follow infants <32 weeks GA for their respiratory needs, which routine evaluations and studies should be performed around discharge, and identifying certain comorbidities with higher risk for long-term complications. The importance of family education was highlighted as a key to successful transition to home. Where consensus was not reached, resource limitations and lack of evidence were the most commonly cited rationales.
Supplementary Material
Acknowledgments
We thank Tregony Simoneau MD (Boston Children’s Hospital), Jonathan M Gaffin MD MMSc (Boston Children’s Hospital), Jonathan Litt MD MPH (Beth Israel Deaconess Medical Center) for reviewing an early version of the Delphi survey.
Funding/Support:
This project was supported by NIH/NHLBI K23 HL136851 (LPH) and the Boston Children’s Hospital Medical Staff Organization’s 2020 House Officer Development Award (JCL).
Neither the NIH nor the Boston Children’s Hospital Medical Staff Organization had a role in the study design, data collection, data analysis, interpretation of the data, writing of the report, or the decision to submit the paper for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the NIH.
Abbreviations
- BPD
Bronchopulmonary dysplasia
- GA
Gestational Age
- IUGR
Intrauterine Growth Restriction
- NICU
Neonatal Intensive Care Unit
- PMA
Post-menstrual age
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest relevant to this article to disclose
References
- [1].Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA 2015;314:1039–51. 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Martin JA, Osterman MJK. Describing the Increase in Preterm Births in the United States, 2014-2016. NCHS Data Brief 2018:1–8. [PubMed] [Google Scholar]
- [3].Collaco JM, McGrath-Morrow SA. Respiratory Phenotypes for Preterm Infants, Children, and Adults: Bronchopulmonary Dysplasia and More. Ann Am Thorac Soc 2018;15:530–8. 10.1513/AnnalsATS.201709-756FR. [DOI] [PubMed] [Google Scholar]
- [4].Abman SH, Collaco JM, Shepherd EG, Keszler M, Cuevas-Guaman M, Welty SE, et al. Interdisciplinary Care of Children with Severe Bronchopulmonary Dysplasia. J Pediatr 2017;181:12–28.e1. 10.1016/j.jpeds.2016.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Beam AL, Fried I, Palmer N, Agniel D, Brat G, Fox K, et al. Estimates of healthcare spending for preterm and low-birthweight infants in a commercially insured population: 2008-2016. J Perinatol 2020;40:1091–9. 10.1038/s41372-020-0635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Levin JC, Beam AL, Fox KP, Mandl KD. Medication Utilization in Children Born Preterm in the First Two Years of Life. J Perinatol 2021;41:1732–8. 10.1038/s41372-021-00930-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Smith VC, Zupancic JAF, McCormick MC, Croen LA, Greene J, Escobar GJ, et al. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. J Pediatr 2004;144:799–803. 10.1016/j.jpeds.2004.03.026. [DOI] [PubMed] [Google Scholar]
- [8].Rhein LM, Konnikova L, McGeachey A, Pruchniewski M, Smith VC. The role of pulmonary follow-up in reducing health care utilization in infants with bronchopulmonary dysplasia. Clin Pediatr (Phila) 2012;51:645–50. 10.1177/0009922812439242. [DOI] [PubMed] [Google Scholar]
- [9].Collaco JM, Kole AJ, Riekert KA, Eakin MN, Okelo SO, McGrath-Morrow SA. Respiratory medication adherence in chronic lung disease of prematurity. Pediatr Pulmonol 2012;47:283–91. 10.1002/ppul.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Duijts L, van Meel ER, Moschino L, Baraldi E, Barnhoorn M, Bramer WM, et al. European Respiratory Society guideline on long-term management of children with bronchopulmonary dysplasia. Eur Respir J 2020;55:1900788. 10.1183/13993003.00788-2019. [DOI] [PubMed] [Google Scholar]
- [11].Cristea AI, Ren CL, Amin R, Eldredge LC, Levin JC, Majmudar PP, et al. Outpatient Respiratory Management of Infants, Children, and Adolescents with Post-Prematurity Respiratory Disease: An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2021;204:e115–33. 10.1164/rccm.202110-2269ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Das A, Cina L, Mathew A, Aziz H, Aly H. Telemedicine, a tool for follow-up of infants discharged from the NICU? Experience from a pilot project. J Perinatol 2020;40:875–80. 10.1038/s41372-020-0593-5. [DOI] [PubMed] [Google Scholar]
- [13].Davis J, Gordon R, Hammond A, Perkins R, Flanagan F, Rabinowitz E, et al. Rapid Implementation of Telehealth Services in a Pediatric Pulmonary Clinic During COVID-19. Pediatrics 2021;148:e2020030494. 10.1542/peds.2020-030494. [DOI] [PubMed] [Google Scholar]
- [14].Ho T, Dukhovny D, Zupancic JAF, Goldmann DA, Horbar JD, Pursley DM. Choosing Wisely in Newborn Medicine: Five Opportunities to Increase Value. Pediatrics 2015;136:e482–489. 10.1542/peds.2015-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–9. 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- [16].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116:1353–60. 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- [19].Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med 2010;182:237–45. 10.1164/rccm.200912-1806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Landry JS, Chan T, Lands L, Menzies D. Long-term impact of bronchopulmonary dysplasia on pulmonary function. Can Respir J 2011;18:265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Levin JC, Sheils CA, Gaffin JM, Hersh CP, Rhein LM, Hayden LP. Lung function trajectories in children with post-prematurity respiratory disease: identifying risk factors for abnormal growth. Respir Res 2021;22:143. 10.1186/s12931-021-01720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pramana IA, Latzin P, Schlapbach LJ, Hafen G, Kuehni CE, Nelle M, et al. Respiratory symptoms in preterm infants: burden of disease in the first year of life. Eur J Med Res 2011;16:223–30. 10.1186/2047-783x-16-5-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Broström EB, Thunqvist P, Adenfelt G, Borling E, Katz-Salamon M. Obstructive lung disease in children with mild to severe BPD. Respir Med 2010;104:362–70. 10.1016/j.rmed.2009.10.008. [DOI] [PubMed] [Google Scholar]
- [24].Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, et al. Pediatric Pulmonary Hypertension. Circulation 2015;132:2037–99. 10.1161/CIR.0000000000000329. [DOI] [PubMed] [Google Scholar]
- [25].Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 2007;120:1260–9. 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- [26].Mourani PM, Abman SH. Pulmonary Hypertension and Vascular Abnormalities in Bronchopulmonary Dysplasia. Clin Perinatol 2015;42:839–55. 10.1016/j.clp.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Radford PJ, Stillwell PC, Blue B, Hertel G. Aspiration Complicating Bronchopulmonary Dysplasia. CHEST 1995;107:185–8. 10.1378/chest.107.1.185. [DOI] [PubMed] [Google Scholar]
- [28].Akangire G, Manimtim W, Nyp MF, Noel-MacDonnell J, Kays AN, Truog WE, et al. Clinical Outcomes among Diagnostic Subgroups of Infants with Severe Bronchopulmonary Dysplasia through 2 Years of Age. Am J Perinatol 2018;35:1376–87. 10.1055/s-0038-1655761. [DOI] [PubMed] [Google Scholar]
- [29].Aucott SW, Watterberg KL, Shaffer ML, Donohue PK, PROPHET Study Group. Do cortisol concentrations predict short-term outcomes in extremely low birth weight infants? Pediatrics 2008;122:775–81. 10.1542/peds.2007-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J Pediatr 2018;197:300–8. 10.1016/j.jpeds.2018.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am J Respir Crit Care Med 2019;200:751–9. 10.1164/rccm.201812-2348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


