Abstract
Spasticity and balance disability are major complications following traumatic brain injury (TBI). Although monoaminergic inputs provide critical adaptive neuromodulations to the motor system, data are not available regarding the levels of monoamines in the brain regions related to motor functions following repetitive blast TBI (bTBI). The objective of this study was to determine if mild, repetitive bTBI results in spasticity/balance deficits and if these are correlated with altered levels of norepinephrine, dopamine, and serotonin in the brain regions related to the motor system. Repetitive bTBI was induced by a blast overpressure wave in male rats on days 1, 4, and 7. Following bTBI, physiological/behavioral tests were conducted and tissues in the central motor system (i.e., motor cortex, locus coeruleus, vestibular nuclei, and lumbar spinal cord) were collected for electrochemical detection of norepinephrine, dopamine, and serotonin by high-performance liquid chromatography. The results showed that norepinephrine was significantly increased in the locus coeruleus and decreased in the vestibular nuclei, while dopamine was significantly decreased in the vestibular nuclei. On the other hand, serotonin was significantly increased in the motor cortex and the lumbar spinal cord. Because these monoamines play important roles in regulating the excitability of neurons, these results suggest that mild, repetitive bTBI-induced dysregulation of monoaminergic inputs in the central motor system could contribute to spasticity and balance disability. This is the first study to report altered levels of multiple monoamines in the central motor system following acute mild, repetitive bTBI.
Keywords: Monoamine, Spasticity, Balance, Traumatic brain injury
1. Introduction
Active duty soldiers are repetitively exposed to explosives in combat theaters such as Iraq and Afghanistan (Shively and Perl, 2012), resulting in blast-induced traumatic brain injury (bTBI). Recent bTBI studies in animal models evaluated alterations in monoamine levels relative to mood and cognitive disabilities (Kawa et al., 2015, 2018). Traumatic brain injury (TBI) is also known to cause a variety of motor disorders, including spasticity, (Bose et al., 2002; Mukherjee and Chakravarty, 2010; Nakase-Richardson et al., 2013; Pasquina et al., 2014) and balance disability (Bose et al., 2013; Fausti et al., 2009; Hoffer et al., 2010) in humans and animals. Since our TBI animals with motor disabilities also revealed systemic monoamine dysregulation, we anticipated that additional factors of bTBI induced monoaminergic alteration would be spasticity, balance, and motor disability. These type of motor disabilities negatively impact their active service and quality of life. Accordingly, it would be important to determine if bTBI also induces motor impairments that correlate with monoamine alterations in strategic motor nuclei such as the motor cortex (MCx), vestibular nuclei (VN), and lumbar spinal cord (LSC). These studies could provide a wider range of understanding of bTBI induced disability. Although the specific etiology of these disabilities is unknown, several studies have shown the efficacy of baclofen in attenuating TBI-induced spasticity in human patients (Francois et al., 2001; Ordia et al., 2002), including that of supraspinal origin (Rifici et al., 1994). Therefore, both spinal and supraspinal levels of dysregulation seems to be involved in TBI-induced spasticity in humans. However, effective treatments for TBI-induced spasticity and balance disability have significant adverse effects (Borrini et al., 2014; Calabro et al., 2014; Cardoso et al., 2014; Motta and Antonello, 2014; Taira et al., 2013). Accordingly, increased knowledge underlying the neuropathology of TBI-induced spasticity and balance disability is urgently needed to provide additional indices of treatment assessments critical for comprehensive tests of new, safe, and effective treatments.
TBI is known to induce spasticity and balance disability in both acute and chronic phases (Bose et al., 2013) and is associated with dysregulation of supraspinal descending pathways such as the corticospinal (CST) and vestibulospinal tracts (Miller et al., 2014; Mukherjee and Chakravarty, 2010; Zong et al., 2016). Activities in these tracts are known to be highly regulated by the action of monoamines in the cerebrum (e.g., motor cortex) and the brain stem (e.g., vestibular nucleus) (Di Mauro et al., 2008; Pompeiano, 1991; Pompeiano et al., 1991; Schuerger and Balaban, 1999). Furthermore, spasticity is known to be influenced by bulbospinal monoamine projections which influence gain control of primary afferent transmission by acting on receptors at presynaptic terminals of interneurons and α-motoneurons in the spinal cord, (D’Amico et al., 2013; Garcia-Ramirez et al., 2014) as well as dendritic excitability via persistent inward current channels (Bennett et al., 1998a, 1998b; Heckmann et al., 2005). Recent comprehensive reviews of monoamines’ influence on the motor system have emphasized a neuromodulatory role of monoamines in enhancing adaptive participation of the motor system in response to dynamic changes in the organism’s experience (Vitrac and Benoit-Marand, 2017).
Following bTBI in rodents, several studies have reported the elevated levels of norepinephrine (NE), dopamine (DA), and serotonin (5-HT) in various regions of the cerebrum and brain stem (Kawa et al., 2015, 2018; Schindler et al., 2017; Tumer et al., 2013) in rodents, while one study showed the reduced level of 5-HT and unaltered DA in the nucleus accumbens of male rats (Sajja et al., 2013). However, these studies were directed toward an increased understanding of cognitive and mood disorders (Kawa et al., 2015; Schindler et al., 2017) and thus motor regions were not investigated. Accordingly, the purpose of the present study was to investigate the alteration of monoamine levels of NE, DA, and 5-HT in the central nervous system responsible for motor and balance functions following repetitive bTBI in rats. We hypothesized that bTBI damages the monoamine neurons and/or their projection fibers, resulting in altered monoaminergic supplies to the MCx, VN, spinal cord motor neurons, and associated regulatory networks. The potential contribution of these changes following TBI are discussed.
2. Results
2.1. Behavioral studies
One day after the third bTBI on day 7 (Fig. 1A), behavioral studies were performed to evaluate spasticity and balance.
Fig. 1.
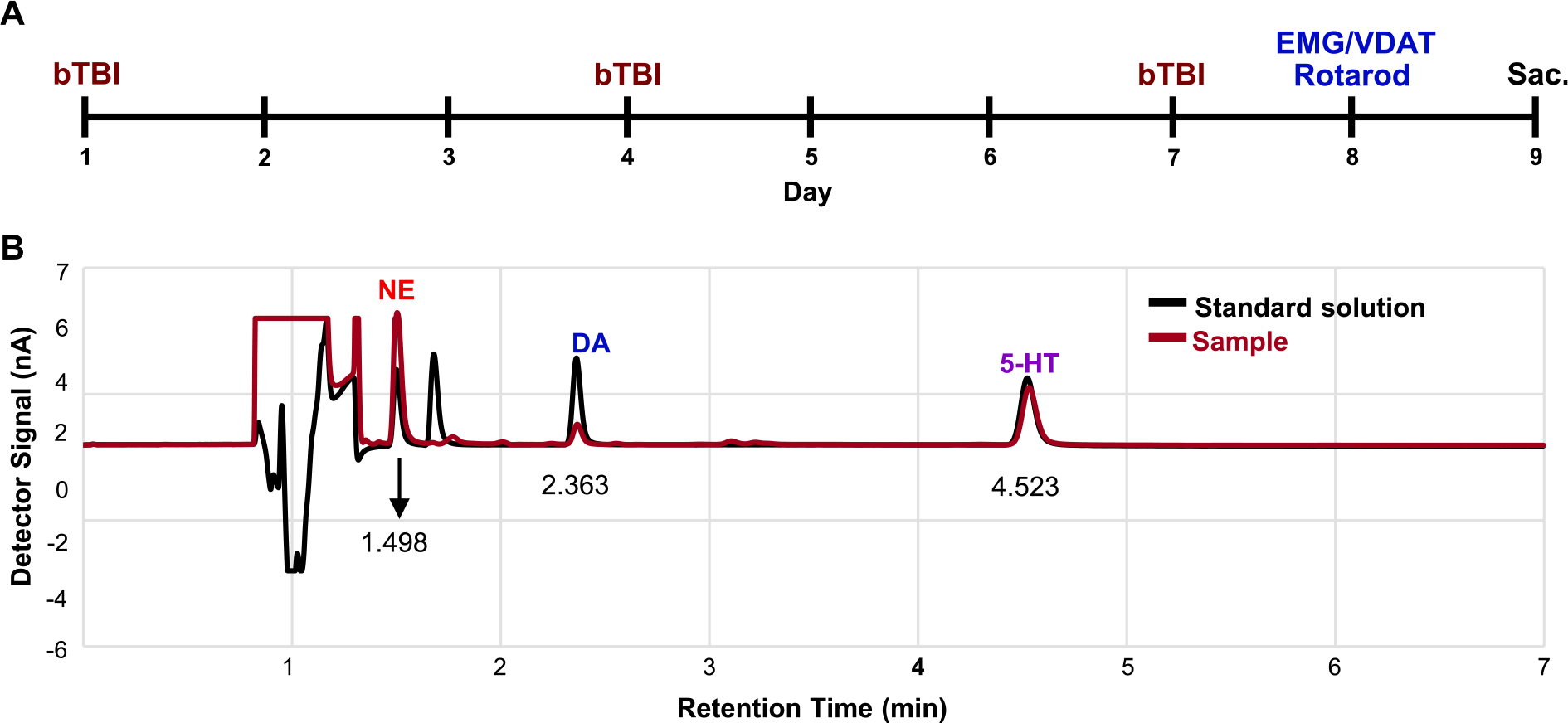
An experimental schedule and representative chromatograms of various monoamines in a standard solution and a brain sample. (A) The experimental schedule of the bTBI animals. Injuries were induced on day 1, 4, and 7 followed by measurement of EMG/VDAT and rotarod tests on day 8. Animals were sacrificed on day 9. (B) The representative chromatograms of HPLC with ECD using the standard solution (black line) and the brain sample (red line). Notice that retention time of detector signal peaks of monoamines in the standard solution and the brain sample was completely overlapped. Sac. = sacrificed.
2.1.1. Electromyography (EMG)
EMG burst discharges that were time-locked to the onset of foot rotation (which induced controlled lengthening of the triceps surae muscles) were observed in the triceps surae muscles in all animals. However, amplitudes of EMG burst discharges time-locked to the onset of the velocity-dependent ankle torque (VDAT) (Fig. 2A) were significantly increased in six of eight test velocities (612°, 350°, 272°, 204°, 136°, and 49° per second) (P < 0.05 and 0.01, Fig. 2B) in bTBI animals compared to data obtained in sham animals. The largest increases were observed at 272°, 204°, 136°, and 49° per second (P < 0.01).
Fig. 2.
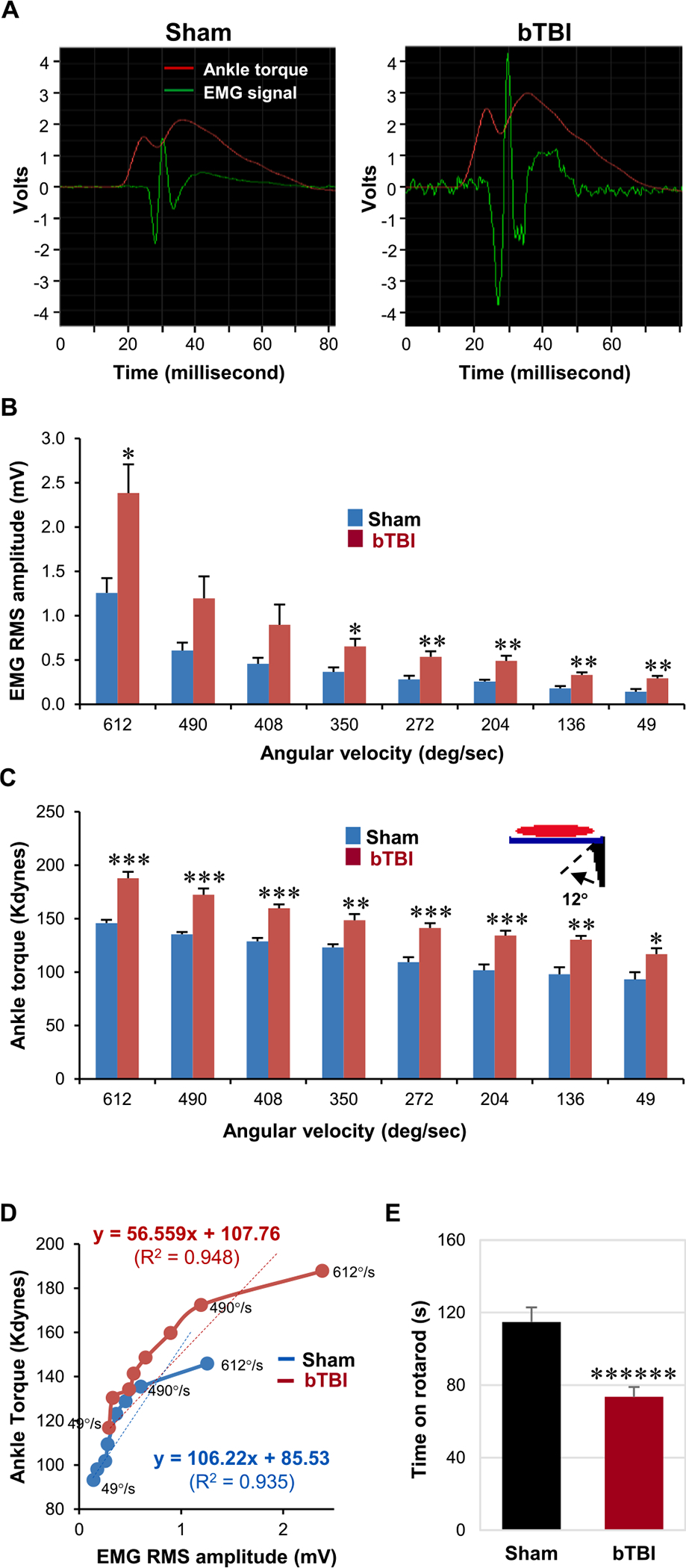
Analysis of bTBI-induced spasticity and balance/motor deficit after acute repetitive bTBI. (A) Representative electromyograms of EMG signals (green) time-locked to the VDAT (red) during stretch reflexes (i.e., dorsiflexion) of the animals in the sham and bTBI groups. Notice the sudden elevation of EMG signals followed by gradual increase in the VDAT in both groups. (B) EMG root mean square (RMS) amplitude during 12°-dorsiflexion at various velocities (612°, 490°, 408°, 350°, 272°, 204°, 136°, and 49° per second). At 612°, 350°, 272°, 204°, 136°, and 49° per second, the EMG RMS amplitude was significantly elevated following injury. (C) VDAT during the dorsiflexion at various velocities. At all tested velocities, the VDAT was significantly elevated following injury. (D) VDAT plotted as a function of EMG RMS amplitude. The slope reflects the efficiency of the neuronal inputs (i.e., EMG) in producing the mechanical outputs (i.e., VDAT). The slope was relatively constant at lower velocities of dorsiflexion but was decreased at higher velocities (beyond 490° per second) in both groups. Regression lines formatted through the initial 7 velocities revealed R2 values of 0.935 and 0.948, respectively, for sham and bTBI groups. (E) Rotarod performance after repetitive bTBI. bTBI animals showed significant balance/motor deficit, compared to age-matched sham animals. Seven independent animals were used per group. Values are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ******p < 0.000001.
2.1.2. VDAT
VDATs recorded in sham animals during 12° foot rotations at eight velocities between 49° and 612° per second revealed 93.13 kdynes at 49° per second with gradual increases to 145.84 kdynes at the highest test velocity. By comparison, VDATs recorded during 12° dorsiflexion were significantly increased in the bTBI group at all eight tested ankle rotation velocities from 49° through 612° per second compared to those in the sham treatment group (P < 0.05, 0.01, and 0.001; Fig. 2C).
2.1.3. VDAT in relation to EMG
VDATs were plotted as a function of the time-locked EMG root mean square amplitudes to more directly evaluate changes in ankle torque with changes in triceps surae neuromuscular excitation, where increases in torque represent relative measures of velocity-dependent increase in muscle stiffness, and EMG amplitudes are sample estimates of the number and synchrony of neuromuscular motor unit excitation. The plots revealed that in both sham and bTBI animals, progressively greater torques were accompanied by progressively greater EMG amplitudes. In the bTBI animal data, the curve revealed a continued growth in both torque and EMG amplitudes, particularly from 350 through 612 deg/sec. These data indicated that, progressively, greater velocity-dependent stiffness and motor unit activations were recruited during the ankle rotations at the higher test velocities in the bTBI animals. Least squares regression analysis of sham and bTBI plots revealed that each displayed relatively linear growth, particularly, during the initial 7 test velocities. Rregression lines formatted through the 8 velocities revealed R2 values of 0.935 and 0.948, respectively, for sham and bTBI plotted curves (Fig. 2D). Collectively, these data (Fig. 2) revealed that significantly increased velocity-dependent ankle torques (active muscle stiffness) and EMG amplitudes (motor unit activations) were recorded in the bTBI animals (Fig. 2B and 2C); these data indicated that, following bTBI, significantly greater active stiffness (muscle activation) and motor unit activations were observed, which was consistent with a clinical condition of spasticity. In addition, the significantly increased values observed at the lowest test velocities indicated that the spasticity was superimposed on a rigidity (stiffness at low velocity).
The decrease in slope could be explained that in sham animals, lower EMGs produced activation of stiffer units than in bTBI (slow motor units). This could suggest that, after injury, activation of progressively faster motor units (which require higher burst frequency for sustained contraction) could have contributed to the decrease in net stiffness/EMG.
2.1.4. Motor coordination/Balance performance studies
Tests for balance scored by walk time on a rotarod revealed a mean walk time of 114.8 ± 8.05 s during the 120-s trial sessions (95.7% completion of the 2-min balance sessions) in sham animals. The mean walk time for bTBI animals was 73.5 ± 5.5 s (61.3% completion of the 2-min balance session), which represented a significant (36.0%) decrease in performance compared to sham animals (P < 0.000001; Fig. 2E).
2.2. Monoamine studies
One day after the behavioral studies, tissues were obtained from the animals to determine monoamine levels in selected regions of the central motor system.
2.2.1. Chromatogram of high-performance liquid chromatography (HPLC) with electrochemical detection (ECD)
A chromatogram of the standard solution containing various monoamines showed that the retention time of the detector signal peaks of NE, DA, and 5-HT were approximately 1.498, 2.363, and 4.523 min, respectively (Fig. 1B, black line). The signal peaks of the monoamines in the experimental samples occurred at the same time periods as those in the standard solution (Fig. 1B, dark red line).
2.2.2. NE levels
Analysis of the HPLC/ECD-derived data for NE levels demonstrated that repetitive bTBI induced significant elevation in the locus coeruleus (LC) and significant reduction in the VN, respectively, compared to levels in the sham treatment group (P < 0.05; Fig. 3A, C). A non-significant (P = 0.184) decrease in NE levels appeared in the MCx (Fig. 3E), whereas no change in NE levels was detected in the LSC or hippocampus (HPC) after repetitive bTBI (Fig. 3F, G).
Fig. 3.
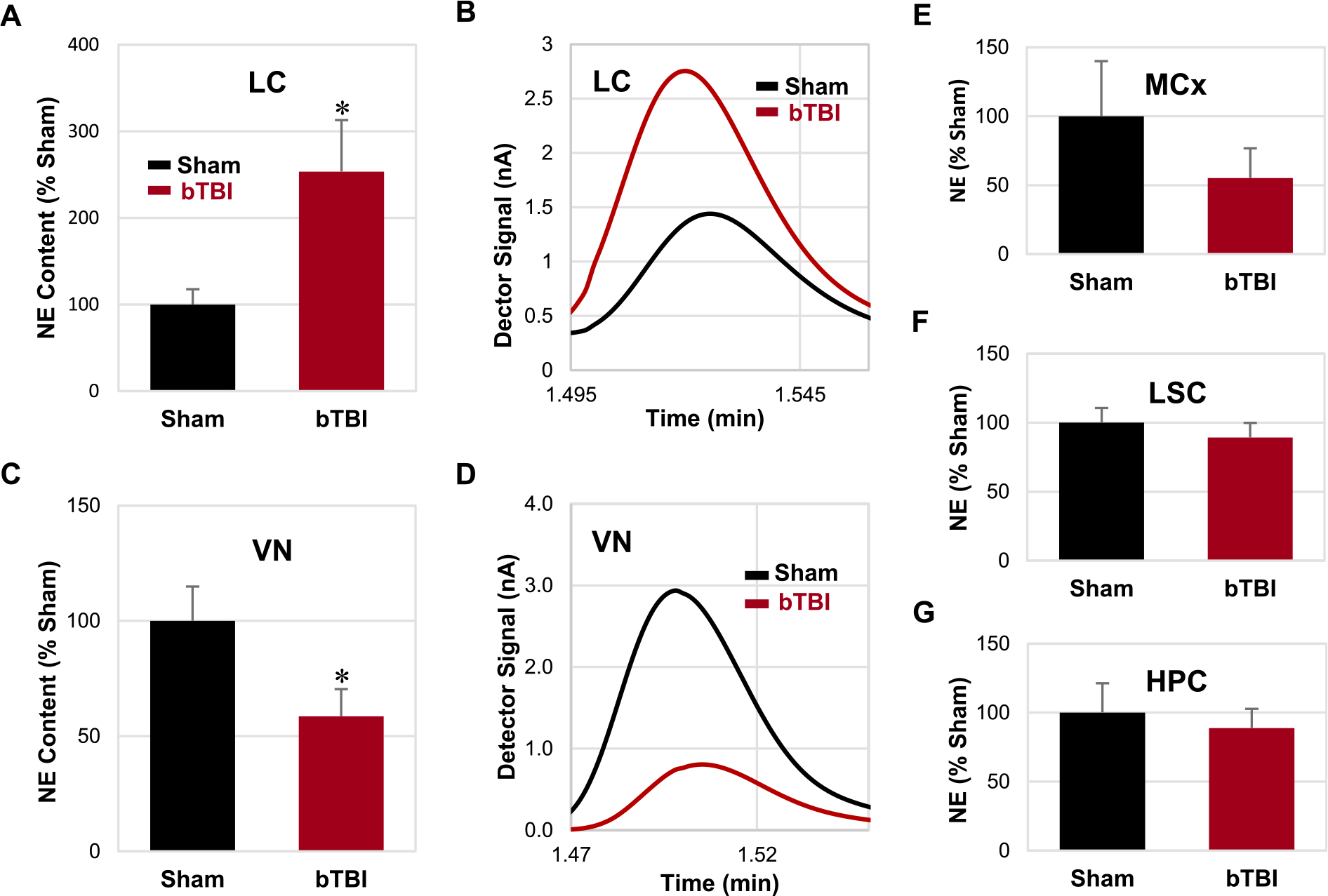
Levels of NE in various central nervous system regions. (A, C) Relative contents of NE were significantly elevated and reduced in the LC and VN, respectively, following repetitive bTBI compared to the sham animals. (B, D) Representative chromatograms of the LC and VN in the sham and bTBI animals. (E-G) No significant difference was detected in the MCx, LSC, and HPC. Bar graphs are shown in the order of statistical significance. Seven independent animals were used per group. Values are mean ± SEM. *p < 0.05.
2.2.3. DA levels
HPLC/ECD data showed that DA levels in the VN was significantly reduced after repetitive bTBI compared to the sham treatment group (P < 0.05; Fig. 4A). Although DA levels were increased and decreased in the HPC and MCx, respectively, after repetitive bTBI (Fig. 4C, D), these changes were not statistically significant (P = 0.117 and 0.169, respectively). No change in DA levels was detected in the LSC or LC after repetitive bTBI (Fig. 4E, F).
Fig. 4.
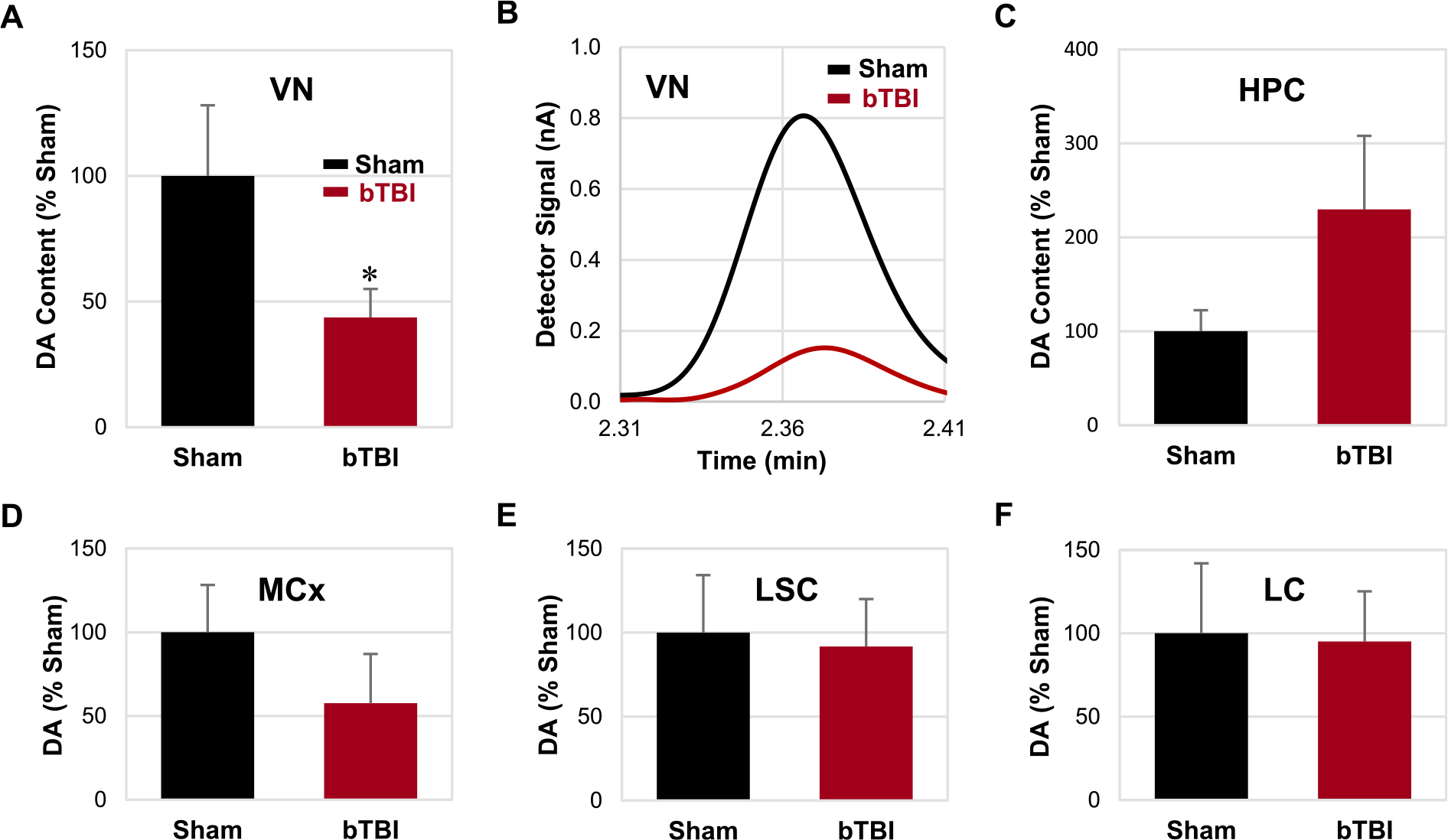
Levels of DA in various central nervous system regions. (A) Relative contents of DA were significantly reduced in the VN following repetitive bTBI compared to the sham animals. (B) Representative chromatograms of the VN in the sham and bTBI animals. (C-F) No significant difference was detected in the HPC, MCx, LSC, and LC. Bar graphs are shown in the order of statistical significance. Seven independent animals were used per group. Values are mean ± SEM. *p < 0.05.
2.2.4. 5-HT levels
HPLC/ECD analysis revealed that the 5-HT levels in the MCx, HPC, and LSC were significantly elevated after repetitive bTBI compared to those in the sham treatment group (P < 0.05; Fig. 5A, C, E). No significant change in 5-HT levels was detected in the VN or LC after repetitive bTBI (Fig. 5F, G) (see Fig. 6).
Fig. 5.
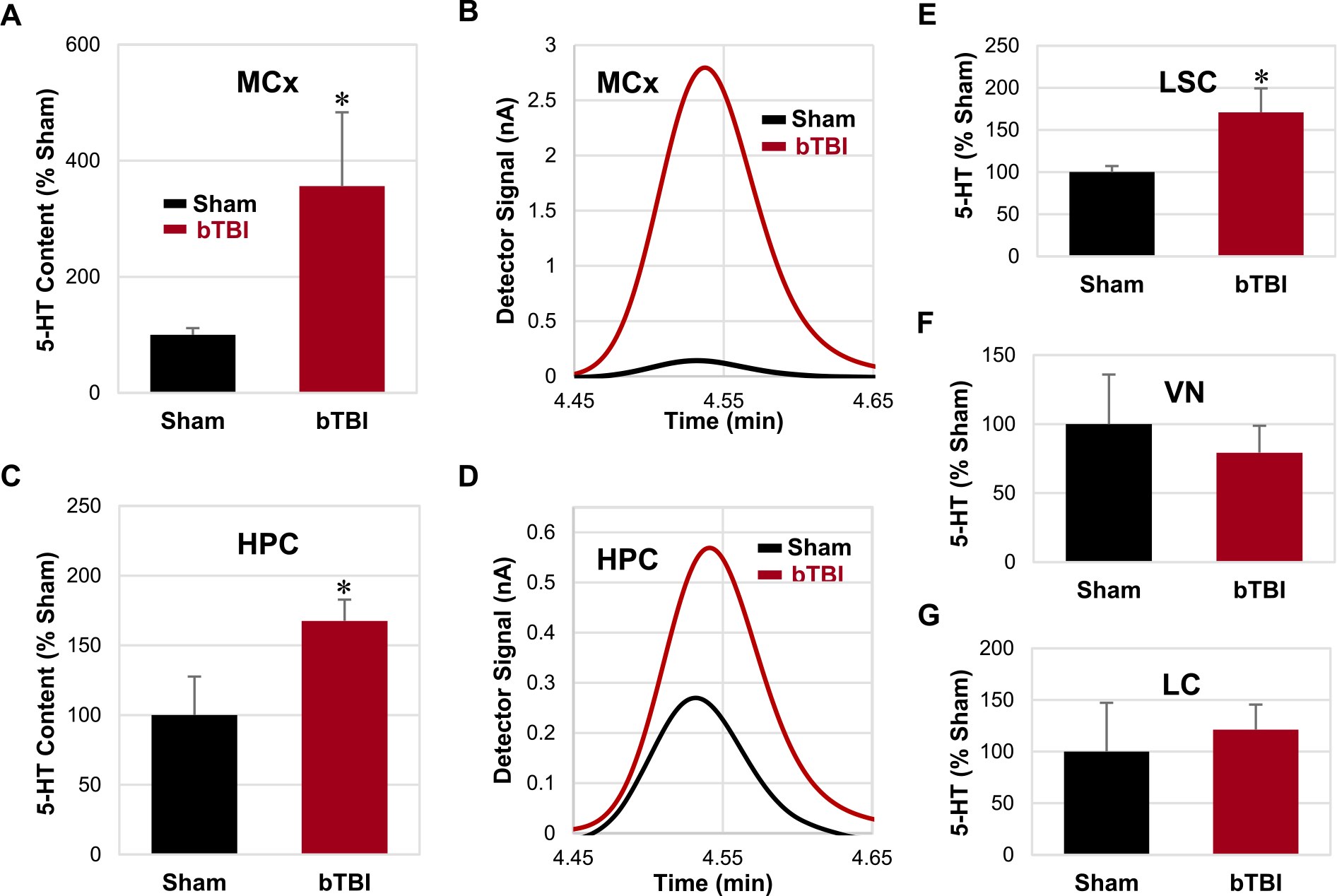
Levels of 5-HT in various central nervous system regions. (A, C, E) Relative contents of 5-HT were significantly elevated in the MCx, HPC, and LSC following repetitive bTBI compared to the sham animals. (B, D) Representative chromatograms of the MCx and HPC in the sham and bTBI animals. (F, G) No significant difference was detected in the VN and LC. Bar graphs are shown in the order of statistical significance. Seven independent animals were used per group. Values are mean ± SEM. *p < 0.05.
Fig. 6.
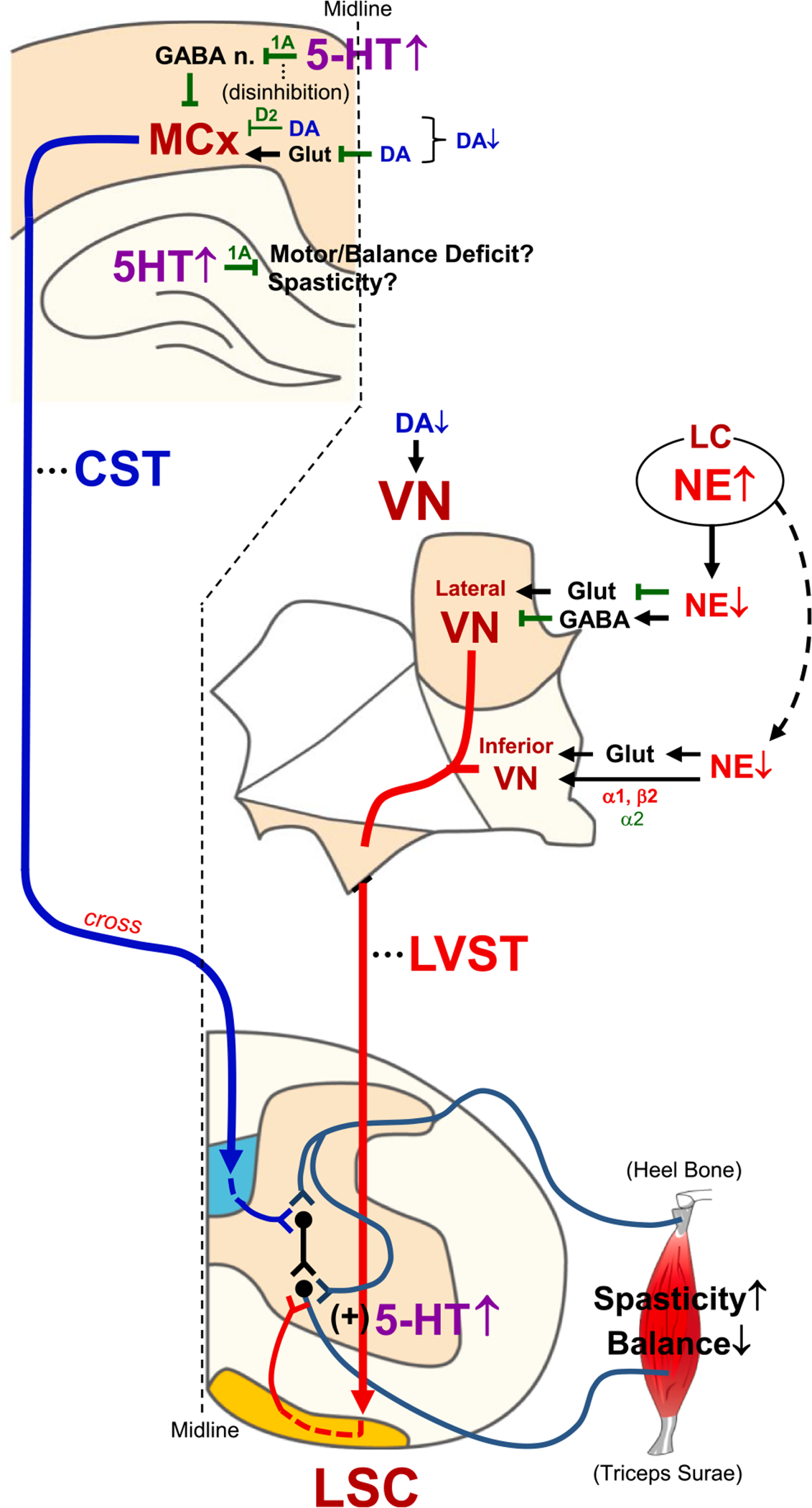
Schematic summary and proposed monoaminergic regulation of spasticity and balance/motor function following repetitive bTBI. Spasticity of hindlimb and balance/motor function are highly regulated by excitability of α-motor neurons in the lumbar spinal cord, which in turn is regulated by various descending tracts from the brain, such as the CST and LVST. Following TBI, LC injury might reduce noradrenergic supply to the VN (Fig. 3C), which in turn could activate and deactivate the lateral VN and inferior VN neurons, respectively, by regulating glutamate- and/or GABA-mediated neuronal activities. In contrast, bTBI-induced reduction of DA in the VN (Fig. 4A) might weaken its stimulatory effect on the downstream tracts. These altered noradrenergic and dopaminergic regulations of the LVST could contribute to elevated spasticity and balance/motor deficits following repetitive bTBI (Fig. 2A–E). On the other hand, 5-HT could be elevated in the MCx following repetitive bTBI (Fig. 5A), which possibly disinhibits CST neurons via activation of 5-HT1A receptor on GABAergic interneurons projecting to pyramidal neurons in the MCx. Because a cortical lesion is known to result in spasticity in rat contralateral hindlimb (Zong et al., 2016), dysregulation of the CST crossing the midline seems to be involved in bTBI-induced spasticity. A moderate reduction of DA in the MCx following bTBI (Fig. 4D) might weaken its inhibitory effect on the pyramidal tract neurons in the Vth layer of the MCx (including CST neurons) via D2 receptor and glutamate. bTBI-induced upregulation of 5-HT in the LSC (Fig. 5E) could produce sustained depolarization and amplification of synaptic inputs to α-motor neurons (Perrier et al., 2013). This monoamine-mediated dysregulation of descending tracts and α-motor neurons might contribute to spasticity and balance/motor disability following repetitive bTBI. Schematics of the central nervous system sections were modified from published materials (Paxinos and Watson, 2009; Watson et al., 2009).
3. Discussion
The present preclinical study detected and quantitated significant hindlimb spasticity and balance/motor disability following acute repetitive bTBI in rats (Fig. 2). In addition, the study revealed significant alterations in NE, DA, and 5-HT levels in selected brain regions and the LSC after bTBI (Figs. 3–5). It is known that monoamines induce changes in excitability, which enhances the adaptive modulation of sensory/motor neural systems. These modulatory processes influence afferent terminal, synaptic, and cellular excitability in the spinal cord (Bennett et al., 1998a, 1998b; Heckmann et al., 2005), VN (Pompeiano, 1991; Pompeiano et al., 1991) and MCx (Vitrac and Benoit-Marand, 2017). Accordingly, these alterations in the monoaminergic levels following repetitive bTBI have potentially important functional implications regarding spasticity and balance disability. It is presumed that spasticity and balance behaviors are interactively influenced because it is likely that alterations in vestibular excitability contribute to both balance and spasticity, and conversely, increased spasticity diminishes the availability and precision of balance control effectors.
3.1. Spasticity measures (VDAT and EMG)
Significantly increased VDATs were recorded in bTBI animals, which were accompanied by significantly increased burst EMGs time-locked to the lengthening of the triceps surae muscles (Fig. 2A–C). These observations indicated that increased ankle torques are likely due to increased muscle stiffness associated, in part, with stretch-evoked muscle activation. This test indirectly measures the changes in motoneuronal activation during controlled lengthening of the triceps surae muscles. The significantly increased stretch-evoked neuromuscular excitation (motoneuronal activation - > EMG) could potentially be accounted for by any or all of several factors, including 1) increased stretch-evoked sensory input (increased stretch receptor input due to increased gamma motor tone in muscle spindles), 2) increased gain of sensory input (decreased presynaptic inhibition), and/or 3) increased postsynaptic response (increased neuronal dendritic plateau potentials and neuronal postsynaptic excitatory potentials). However, the higher start position of the bTBI VDAT/EMG plot (Fig. 2D) suggests that the stretch evoked (phasic) input was also superimposed on a significantly increased tonic input to the motoneurons. The increased tonic input from tonic stretch reflex or increased descending motor inputs would increase the gain of the stretch evoked responses that would, in turn, increase the activation (stiffness) of the triceps surae muscles. Accordingly, the measures reflect that the significantly increased VDATs are associated with increased tonic and phasic increases in triceps surae muscle activation. It is known that a principle contribution of monoamine modulation is tonic and phasic modulation of inputs to motoneurons. We posit that these spasticity changes are, in part, consistent with the significant alterations in monoamine levels observed in the spinal cord, VN, and MCx in bTBI animals.
3.2. Muscle spindles and spinal cord
Muscle stretch is sensed by intrinsic muscle spindles that have complex sensory/motor structures composed of intrafusal muscle fibers and stretch sensitive equatorial regions innervated by group 1a and II afferents. The sensitivity of these receptors is governed by the activity of gamma motoneurons that innervate contractile components of the spindles. These receptors and afferents provide tonic and phasic information regarding muscle length, and these properties are differentially regulated by static and dynamic gamma motoneuron activity (Ellaway et al., 2015). It is known that the activity of gamma motoneurons can be modulated by 5-HT and NE. 5-HT is predominantly excitatory to gamma motoneurons (Ahlman et al., 1971; Ellaway and Trott, 1975), whereas NE is inhibitory, although its actions are more complex (Bennett et al., 1996a, 1996b). 5-HT enhanced the resting activity of gamma motoneurons and facilitated activation of the muscle afferents (Jankowska et al., 1998). Accordingly, the increased tonic and phasic responses observed in the VDAT and EMG recordings could, in part, be accounted for by the significantly increased 5-HT levels observed in the LSC of bTBI animals (Fig. 5E).
Muscles are activated by increased activity of innervating spinal motoneurons. The monoamines 5-HT, NE, and DA are known to provide significant modulation of the excitability of the spinal motoneurons. 5-HT innervation to the LSC is through fiber projections from the raphe nuclei in the brainstem. Its modulatory role on motoneurons is exerted on the somato-dendritic compartments of motoneurons that are densely innervated by serotonergic synaptic boutons. In addition, several 5-HT receptors (including 5-HT1A, 5-HT1B, 5-HT1D, 5-HT2A, 5-HT2B, 5-HT2C and 5-HT5A) are expressed in the membrane of motoneurons. Activation of these receptors has significant physiological consequences (Wei et al., 2014) and increases the excitability of motoneurons through modulation of several classes of ion channels (Perrier et al., 2013). Collectively, 5-HT depolarizes motoneurons towards their activation threshold by inhibiting leak conductances and promoting hyperpolarization-activated cationic current. 5-HT also increases motoneuron firing frequency by inhibiting small Ca2+-activated K+ conductance, which produces medium after-hyperpolarization following action potentials. 5-HT modulation of voltage sensitive Ca2+ and Na+ conductances also promotes persistent inward currents that produce sustained depolarization and amplification of synaptic inputs. Under pathological conditions, such as after a spinal cord injury, the promotion of persistent inward currents by 5-HT and/or the overexpression of autoactive serotonergic receptors may contribute to motoneuronal excitability, muscle spasms, and spasticity, and hence, to impairment of stereotyped motor behaviors such as locomotion. Accordingly, significantly increased 5-HT expression in the LSC of bTBI animals (Fig. 5E) could potentially have influenced multiple processes leading to increased motoneuronal excitability.
3.3. LC in spasticity and motor disability
NA projections from the LC critically regulate excitability of spinal cord reflexes, vestibulo-spinal reflexes, and motor cortex gait coordination (Di Mauro et al., 2008; Schiemann et al., 2015; Schuerger and Balaban, 1993). TBI-induced spasticity has been proposed to be related to the progressive loss of noradrenergic (and also GABA-ergic) inhibition which significantly contributes to the progressive development of inappropriate muscle activation during locomotion (Thompson et al., 1992, 1993, 1998, 2001a). Further, we showed that spasticity develops over a time course, which is mirrored by the loss of rate-dependent depression in the reflex pathways that serve the spastic muscles in rats (Bose et al., 2012; Thompson et al., 1992, 1993, 1998) and in humans (Thompson et al., 2001b; Trimble et al., 2001). Monoaminergic system is an important contributor to this reflex pathway. Rate-depression is one of three fundamental presynaptic processes that controls reflex magnitude elicited by repetitive input to motoneurons. Facilitation and potentiation are the other two processes (Mendell, 1984). Rate-depression is known to utilize both pre-synaptic and post-synaptic processes and two neurotransmitter systems known to play critical roles in the modulation of segmental reflex modulation are γ-aminobutyric acid (GABA) and NE. We have observed that the rate-dependent inhibition and VDAT are profoundly influenced by GABAb-specific agents (Wang et al., 2002) and NE-specific lesions (Bose, 2001). Specifically, L-baclofen (which acts upon GABAb segmental circuitry) increased rate-dependent inhibition and decreased VDAT (improved spasticity), whereas selective neurotoxic lesions of NE fibers produced non-specific increase in reflex excitability and elevated ankle torque (spasticity) as we have seen in SCI or TBI. Therefore, significantly elevated NE level in the LC following bTBI in the present study (Fig. 3A) could affect both spasticity and motor/balance performance (Fig. 2A–E) via these mechanisms.
3.4. VN in spasticity and balance disability
The vestibular system has been reported to be a signature site of injury following bTBI (Fausti et al., 2009; Hoffer et al., 2010; Scherer and Schubert, 2009). The vestibular system comprises the semicircular canals and otolithic organs, which provide sensory information to the brain regarding head position and acceleration. In addition, it provides postural motor signals to the eyes, head, and spinal motor systems. Vestibular injury can result in dysregulated projections that alter muscle tone and decrease balance stability. In combination with the sensory input regarding head position, the VN receive significant monoamine innervation that alters vestibulo-motor gain, enhancing the adaptive regulation of balance function. Because the bTBI animals revealed significantly decreased levels of NE and DA in the VN (Fig. 3C and Fig. 4A), the discussion will address these findings and how these potentially relate to the observed behavioral findings.
3.4.1. Role of NE in the VN
Noradrenergic innervation to the lateral VN is derived from the ventral portion of the caudal two-thirds of the ipsilateral LC. This nucleus has been reported to be vulnerable to injury induced by closed-head TBI (Bose et al., 2013), which could affect noradrenergic innervation to multiple brain regions. A high density of fibers immunopositive for DA beta-hydroxylase were found in the dorsal and ventral portions of the lateral VN, which formed the dense plexus on the somata and the proximal dendrites of the lateral VN neurons; the more densely innervated vestibular regions provide significant projections to the spinal cord (Schuerger and Balaban, 1999). Noradrenergic receptor (NA-R) α1, α2, and β1 are expressed throughout the VN, and different regulatory roles have been reported for NE in each subnucleus (Barresi et al., 2014). The in vivo action of NE has been observed to primarily inhibit gating of the vestibular neurons (Licata et al., 1993) via enhancement of inhibition by GABA (Di Mauro et al., 2008). In the rat lateral VN, NE was also observed to predominantly inhibit glutamate-induced neuronal activation (Barresi et al., 2009). In the inferior VN and medial VN, NE predominantly activates neurons via NAα1-R and NAβ2-R, whereas it inhibits them via NAα2-R (Peng et al., 2016; Podda et al., 2001). Among these vestibular subnuclei, the lateral VN and inferior VN are directly involved in the regulation of limb skeletal muscle activity via the lateral vestibulospinal tract (LVST), while other subnuclei (i.e., superior and medial VN) seem to be less involved (Corneil and Camp, 2018). Therefore, regulation of LVST excitability is integrated with glutamate and GABA (Barresi et al., 2009). Here, GABA-mediated inhibition of vestibular neurons is regulated by NE via NAα2-R and NAβ-R in rats (Di Mauro et al., 2008). Because NE was significantly reduced in the VN after repetitive bTBI in the present study (Fig. 3C), this complex pattern of noradrenergic regulation of the LVST appears to be significantly altered following the injuries, possibly contributing to bTBI-induced spasticity (Fig. 2A–D). Considering the overall inhibitory effects of NE on vestibular neurons (Licata et al., 1993), our observations of significantly decreased NE levels in the VN could suggest a release of tonic vestibulo-spinal excitatory input to spinal motor neurons.
Although it is difficult to interpret the resultant effects on balance function, in an intratympanic gentamicin-induced balance disorder model in male rats, the NE content in the VN were significantly elevated 3 days after the treatment (Zhai et al., 2019). Therefore, we cannot exclude the possibility that bTBI-induced significant downregulation of NE in the VN (Fig. 3C) was a compensatory response to impaired balance/motor function (Fig. 2E). Unfortunately, no/few studies have examined a role of vestibular NE in regulating spasticity. It is desired that future studies investigate this research topic.
3.4.2. Role of DA in the VN
The ventral tegmental area and substantia nigra neurons provide DA innervation to multiple brain regions (Hosp et al., 2011; Hosp and Luft, 2013). Previous studies suggested that injury-induced alterations in DA expression could be biphasic with variable patterns in different brain regions and time periods following TBI. In rodent striatum, DA expressions were observed as consistently reduced after controlled cortical impact injury and fluid percussion injury in acute and chronic time frames (Chen et al., 2015; Shin et al., 2011; Wagner et al., 2005, 2009; Xu et al., 2016). However, in other studies, DA expression levels were moderately elevated in the substantia nigra 4 weeks following fluid percussion injury in rats (Liu et al., 2017; van Bregt et al., 2012). These studies suggest that the bioavailability of DA in the nigrostriatal system could be biphasic following TBI. However, few studies have examined DA expression levels in the downstream substrate centers such as the VN. Nonetheless, a previous study showed that levodopa (direct precursor of DA) significantly increased vestibular-evoked myogenic potentials of the sternocleidomastoid muscle in patients with Parkinson’s disease (Potter-Nerger et al., 2012), potentially suggesting an excitatory role for DA in the VN. Accordingly, application of this assumption to the neurons of the LVST suggests that bTBI-induced significant down-regulation of DA in the VN in the present study (Fig. 4A) might have weakened the stimulation of this tract to maintain balance by regulating the body/limb muscles (McCall et al., 2017). The evidence of postural control by the dopaminergic system supports this notion (Herbin et al., 2016). On the other hand, it seems likely that spasticity is not caused by abnormal DA system (Ricciardi et al., 2018). Collectively, significantly altered DA and NE levels in the VN were consistent with decreased balance/motor performance of bTBI animals on the rotorod (Fig. 2E).
3.5. MCx in spasticity and balance disability
The MCx plays a major role in learning and execution of voluntary motor control (Ebbesen and Brecht, 2017; Ebbesen et al., 2018). The cortical neurons are organized in six layers using a variety of interneuronal/pyramidal projection cell types and interconnected local networks to process and transfer information. The cortical neurons receive multiple inputs from other cortical and subcortical structures, particularly the thalamus (Brown and Teskey, 2014). The thalamo-cortical projections reach a small band of pyramidal shaped neurons in the IVth and Vth layers. The Vth layer pyramidal cells (upper motoneurons) send long efferent projections to brainstem and spinal cord motoneurons (lower motoneurons). Approximately 20% of the interconnecting interneurons are GABAergic inhibitory interneurons. The cortex reveals a somatotopic organization such that neuronal clusters receive somatotopically specific input and these neurons, in turn, project to somatotopically discrete pools of lower motoneurons (Asanuma and Rosen, 1972; Brown and Teskey, 2014; Neafsey et al., 1986; Thompson and Fernandex, 1975). In addition, the cortical neurons receive vast information relayed from other cortical regions that process visual, auditory, olfactory, and proprioceptive information. The selection of appropriate behavioral (motor) responses consequent to the environmental contextual inputs requires the cortical networks to integrate vast amounts of information to guide voluntary motor behavior. Cortical information processing benefits from the influence of monoamine neuromodulators that prioritize the signal versus the noise to increase the efficiency and clarity of behavior selection. Collectively, the monoamines enhance the adaptive prioritization of information processing and selective upregulation of task-appropriate sets of cortical neurons (Vitrac and Benoit-Marand, 2017). Following bTBI, animals revealed dysregulation of these monoamines in the MCx including significantly increased expression levels of 5-HT (Fig. 5A).
3.5.1. Role of 5-HT in the MCx
Serotonergic innervation to the MCx originates from the dorsal and median raphe nuclei and is critically involved in cortical function. Acting on presynaptic and postsynaptic receptors, 5-HT is involved in impulse control and motor function by modulating the activity of a variety of cortical neurons and the release of glutamate, GABA, acetylcholine, and DA. A variety of 5-HT receptor subtypes (5-HT1A, 5-HT1B, 5-HT2A, and 5-HT3) are used to modulate the excitability and the discharge rates of cortical neurons. Particularly, 5-HT1A and 5-HT2A receptors are key effectors. 5-HT1A receptors can hyperpolarize pyramidal neurons through opening G protein-coupled inward rectifying K+ channels (Jeong et al., 2001). This influence is followed by a reduction in the firing activity of the pyramidal neurons. Conversely, the activation of 5-HT2A depolarizes neurons, thereby increasing their excitability. 5-HT can also excite (via 5-HT2A and 5-HT3 receptors) or inhibit (via 5-HT1A receptor) GABA interneurons to modulate GABA inhibitory inputs onto pyramidal neurons. The particular mechanisms of selection of specific receptor effects are yet to be identified (Celada et al., 2013).
Following various modalities of TBI, monophasic patterns of 5-HT alteration have emerged from studies in the cerebral cortex. The predominant 5-HT innervation to the forebrain has been reported to project from the rostral raphe complex including the dorsal raphe nucleus (Hornung, 2003). In this nucleus, mRNA of the rate-limiting enzyme for 5-HT synthesis (i.e., tryptophan hydroxylase) was significantly elevated 2 h following bTBI in rats (Kawa et al., 2015). In agreement with this, the extracellular 5-HT expression levels in the cerebral cortex were reported to be significantly elevated 10–90 min after moderate FPI (Busto et al., 1997). Furthermore, our recent work demonstrated a significant elevation of 5-HT fiber density in the cerebral cortex 2 weeks following mild closed-head TBI in rats (Mustafa et al., 2016). In the present study, 5-HT expression levels were significantly elevated in the MCx 2 days following acute repetitive bTBI (i.e., 8 days following the initial injury) (Fig. 5A). Consistent with observed spasticity, upregulation of 5-HT in the MCx could increase the excitability (disinhibition) of CST neurons in the Vth layer. In the rat prefrontal cortex, by predominantly binding to 5-HT1A receptor, 5-HT inhibits fast-spiking GABAergic interneurons that project to pyramidal neurons, resulting in disinhibition (Llado-Pelfort et al., 2010; Puig et al., 2010). On the other hand, the connections of fast-spiking GABAergic interneurons to the retrogradely labeled neurons of the CST in the Vth layer of the MCx have been shown in rats (Tanaka et al., 2011). Therefore, if a mechanism similar to that in the prefrontal cortex is applied to the CST neurons in the Vth layer of the MCx, repetitive bTBI-induced upregulation of 5-HT in the MCx (Fig. 5A) might have disinhibited the CST neurons via 5-HT1A receptor to regulate spinal stretch reflex and spasticity (Mukherjee and Chakravarty, 2010), which could also affect balance and motor function (Fig. 2A–E).
Because spasticity and balance/motor impairments (but not locomotor dysfunction) were induced when cortical 5-HT was abnormally low in certain pathological conditions (Dentel et al., 2013; Lehmann et al., 2000; Mizoguchi et al., 2002a), bTBI-induced significant upregulation of 5-HT in the MCx in the present study (Fig. 5A) might be a compensatory response to attenuate injury-induced spasticity and balance/motor impairments (Fig. 2A–E).
3.5.2. Role of DA in the MCx
DA also plays a critical role in regulating the excitability of cells in the MCx. The rat primary MCx receives predominant DA innervation from the ipsilateral rostrolateral ventral tegmental area (~73%), with the lesser innervations from the contralateral ventral tegmental area (~12%) and ipsilateral rostromedial substantia nigra (~15%) (Hosp et al., 2011; Hosp and Luft, 2013). In anesthetized rats, the exogenous application of DA (0.1 M, 30 s) significantly reduced the firing rate of virtually all pyramidal tract neurons in the Vth layer of the MCx, which was neutralized by co-application of the D2 receptor antagonist. In addition, glutamate-induced increases in the firing rate of these neurons were normalized by co-application of DA (Awenowicz and Porter, 2002). Therefore, the overall function of DA in the MCx appears to be inhibitory. MCx output signals are transmitted through several descending tracts (e.g., CST and dorsal reticulospinal tracts) that collectively influence peripheral and central regulation of muscle tone (Andrews et al., 1973; Mukherjee and Chakravarty, 2010). Eleven to eighteen days after controlled cortical impact injury, performance in the Morris water maze, which requires a significant degree of motor function in addition to spatial learning and memory, was observed as significantly diminished (Kline et al., 2001, 2004; Monaco et al., 2014; Olsen et al., 2012). A unilateral lesion in the MCx resulted in spasticity with elevated Hoffmann’s reflex in the contralateral hindlimb of rats (Zong et al., 2016). Accordingly, injuries to the MCx appear to be associated with spasticity following TBI. Furthermore, DA expression levels in the cortex are reported to be significantly reduced 1 h to 2 weeks after FPI in rats (McIntosh et al., 1994). Consistent with these studies, in the present study, repetitive bTBI induced moderate reduction of DA in the MCx (Fig. 4D) and resulted in spasticity and balance/motor dysfunction (Fig. 2A–E). Importantly, stress reduced DA in the cortex of male rats and worsened rotarod performance (Mizoguchi et al., 2003), which was significantly attenuated by administration of D1 agonist (Mizoguchi et al., 2002b). Furthermore, DA agonist is known to improve motor performances of Parkinson’s Disease patients (Lucetti et al., 2007). Therefore, bTBI-induced reduction of DA in the MCx (Fig. 4D) might have contributed to spasticity and balance/motor dysfunction (Fig. 2A–E).
Our previous work and other studies have shown various severities of spasticity in the limb muscles following closed-head TBI in humans and animals (Bose et al., 2013; Gracies et al., 2015; Jang et al., 2004; Lorentzen et al., 2012; Mas et al., 2018; Yablon et al., 1996), which has been thought to occur due to injury-induced malfunction of the upper motor neurons (Botte et al., 1988). In animals and patients with TBI, this stretch reflex excitability (i.e., spasticity) has been categorized into tonic (or static) and dynamic components based on the reflex properties where the latter is thought to reflect the muscle spindle sensitivity to the lengthening velocity of muscle (Bose et al., 2002, 2013, 2012; Schmit and Rymer, 2001). In the present study, significant elevation of spastic measures (i.e., VDATs), which include both tonic and dynamic components of the stretch reflex, was observed at all tested ankle angular velocities following acute repetitive bTBI compared with sham animals (Fig. 2C). These results are similar to those following cervical spinal cord injury (Hou et al., 2014), while partly different from those following thoracic spinal cord injury in which the tonic component of the stretch reflex at lower angular velocities was not significantly altered (Bose et al., 2002, 2012). On the other hand, EMG amplitudes were significantly elevated at the lower angular velocities of ankle dorsiflexion (49°–350° per second) as well as the highest one (612° per second) following acute repetitive bTBI (Fig. 2B), indicating increased resting muscle tone after injuries. However, the overall significance of the increase in EMG signals following repetitive bTBI was lower than that in the VDATs at most angular velocities with non-significant increases at 490° and 408° per second (Fig. 2B and C), suggesting that the neuronal signal inputs were amplified the by the mechanical component of muscle contraction. Further analysis of the ankle torque–EMG curve revealed that this amplifying function gradually deteriorated as the velocity increased beyond 490° per second in both normal and bTBI animals (Fig. 2D). These increases in EMG signals during stretch reflexes seem to reflect dysregulated control of α-motoneurons by the supraspinal descending tracts following repetitive bTBI. Overall, these results are supported by our previous work in which lower limb spasticity was detected following closed-head TBI in rats (Bose et al., 2013).
3.6. HPC in spasticity and balance/motor disability
In the present study, the level of 5-HT was significantly elevated in the HPC (Fig. 5C), while spasticity and balance/motor deficits were detected following bTBI (Fig. 2A–E). Therefore, possible roles of hippocampal 5-HT in regulating spasticity and balance/motor function following bTBI are discussed below.
3.6.1. Role of hippocampal 5-HT in spasticity
To date, no study has determined a role(s) of hippocampal 5-HT in regulating spasticity. However, some studies gave us a hint to understand its possible role in attenuating pathology-induced spasticity. In human amyotrophic lateral sclerosis patients, reduction of 5-HT fiber density was observed in the dentate gyrus of HPC (Dentel et al., 2013). In another study, activation of 5-HT2A receptors on the spinal motor neurons reduced a spasticity measure via a PKC-dependent signaling pathway following spinal cord injury in rats (Bos et al., 2013). Thus, although the precise mechanism is unknown, we cannot exclude the possibility that bTBI-induced significant upregulation of 5-HT in the HPC in the present study (Fig. 5C) could be a compensatory response to attenuate injury-induced spasticity (Fig. 2A–D).
3.6.2. Role of hippocampal 5-HT in balance/motor disability
Few studies have examined a direct role of hippocampal 5-HT in balance function. Nonetheless, several studies have shown that intraperitoneal injection of 5-HT1A receptor agonists significantly attenuated TBI-induced neuronal cell loss in the hippocampal CA3 subregion, cognitive/motor dysfunction (measured by Morris water maze), and balance/motor deficit (measured by beam balance) in rats (Kline et al., 2010; Phelps et al., 2017). Hence, there is a possibility that a neuroprotective role of 5-HT in the HPC contributes to attenuation of TBI-induced balance/motor deficit. These studies suggest that bTBI-induced significant upregulation of 5-HT in the HPC in the present study (Fig. 5C) might be a compensatory response to attenuate injury-induced hippocampal neuronal cell loss and balance/motor dysfunction (Fig. 2E).
3.7. Gender difference
Few studies have reported the gender difference in altered motor function and monoamine levels following bTBI in rats. Nonetheless, no clear difference in a vestibular response (righting reflex) was observed between male and female mice following mild bTBI (Russell et al., 2018). This study also showed different stress-induced responses of the HPA axis among male and female animals after bTBI. Following bTBI, the percentage of stress-induced activation of corticotropin-releasing factor (CRF)-immunoreactive neurons in the paraventricular nucleus (PVN) of hypothalamus was significantly decreased in male mice, while the responses of female mice were opposite (Russell et al., 2018). Since it is likely that CRF cells in the PVN are regulated by noradrenergic innervation from A2 cell group (Liposits et al., 1986; Plotsky et al., 1989), altered noradrenergic supply from the A2 cell group to the CRF neurons might have affected activation status of CRF neurons in this study. On the other hand, stress-induced serum corticosterone was significantly elevated following bTBI in male mice although opposite responses were observed in female mice (Russell et al., 2018). Therefore, under stressed condition, these gender differences might affect behavioral outcomes of balance/motor assessments (e.g. rotarod test) via altered anxiety following bTBI. Further studies are desired for the paradoxical responses of the stress-induced HPA axis (activation status of CRF neurons versus serum CORT levels) among male and female mice following bTBI.
3.8. Monoamine expressions and time points after blast injury
Depending on a time point after injury, the effects of bTBI on monoamine expressions might differ. Following a single bTBI of male rats, some similar and different results from the present study were shown (Kawa et al., 2015). In this study, the levels of tyrosine hydroxylase mRNA in the LC were significantly elevated 2 h after bTBI, which agrees with elevated NE level in the LC in the present study (Fig. 3A) in spite of different time point. In the dorsal HPC, the DA levels were significantly elevated 1 day after injury, while they went back to normal level within 7 days. In agreement with this study, the DA level was not significantly altered (although slightly elevated) in the dorsal HPC 8 days after the initial bTBI in the present study. However, slightly elevated level of hippocampal DA (Fig. 4C) indicates that the second and third bTBI (on day 4 and 7) might have maintained the dopaminergic response to the HPC longer. Finally, the 5-HT levels were not significantly altered (although elevated by 35% and 27%, respectively) in the dorsal HPC 1 and 7 days following a single bTBI (Kawa et al., 2015). In contrast, the present study showed significantly elevated 5-HT in the dorsal HPC (Fig. 5C). Therefore, the second and third bTBI (on day 4 and 7) might have amplified the serotonergic response to the initial injury.
4. Conclusion
As discussed above, altered monoamine levels were observed in the central nervous system following mild repetitive bTBI, which was correlated with measures of significant lower limb spasticity and balance/motor impairment. Although optimal therapeutic methods are not yet available for TBI-induced spasticity and balance deficit by regulating monoaminergic systems, in the last few decades, a number of studies have evaluated the efficacy of baclofen on spasticity (Francois et al., 2001; Ordia et al., 2002; Perez-Arredondo et al., 2016; Rifici et al., 1994; Wang et al., 2002). Because the GABAergic system could be a derivative of monoamine regulation, monoaminergic treatments could potentially be therapeutic options for TBI-induced behavioral deficits. To the best of our knowledge, this is the first study to show altered bioavailability of various monoamines in the cerebrum, brain stem, and spinal cord following repetitive bTBI. These findings provide novel information of dysregulated supplies of various monoamines to a variety of brain regions following this modality of TBI, which could contribute to a better understanding of TBI neurobiology as well as the development of new therapeutic alternatives.
5. Experimental procedure
This study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
5.1. Animals
This study was performed on animals (rodents), and the experimental protocols were approved by the Institutional Animal Care and Use Committee of the North Florida/South Georgia Veterans Health System and University of Florida before initiating the project. Fourteen adult (12–14 weeks old, 250–280 g) male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were randomly assigned to the bTBI and sham treatment groups (n = 7 per group) and were coded to blind all experimenters (except a senior investigator) to injury or sham allocation. Each pair of rats was kept in a standard cage with controlled humidity and temperature under a 12-h light–dark cycle at an American Association for Laboratory Animal Science-accredited facility. All experiments were conducted during normal work hours (8 am–5 pm). Food and water were ad libitum. Maximal efforts were made to reduce the number of animals per group. Animals were handled with care and compassion by experts. They were monitored to minimize any sign of pain or discomfort after the treatment and during experiments. Although buprenorphine (Reckitt Benckiser Pharmaceuticals, Richmond, VA, USA) was injected for postoperative pain care, no analgesic was used during behavioral experiments because these experiments do not inflict pain on animals.
5.2. bTBI
Brain injury was induced by a blast overpressure wave to each animal in the bTBI group on days 1, 4, and 7 (Fig. 1A), as previously described (Toklu et al., 2018). This protocol was optimized based on the previous studies that showed a pattern of recovery within 3 days following various intensities, frequencies, and the time course of injuries in humans and animals (Larres et al., 2016; Wang et al., 2011). This protocol has been shown to result in a variety of neurobiological complications, including neurological deficits, edema, disruption of the blood–brain barrier, and upregulation of cell injury/inflammatory markers in the related brain regions (Toklu et al., 2018). Briefly, under anesthesia with 3% isoflurane (Baxter International, Deerfield, IL, USA), the animal was inserted into the polyvinyl chloride animal holder in a prone position exposing only his head until the head was positioned under the pressure sensor inside the horizontal shock tube. To decrease the surface reflection of blast waves and formation of secondary waves that could possibly exacerbate the injury, the head was placed on a flexible mesh surface. Each animal in the bTBI group was transiently exposed to a blast wave (2.0–2.5 ms, peak pressure of 30 lb-force per square inch) that produced a blast waveform with positive pressure followed by negative pressure, whereas each animal in the sham group received only anesthesia. After it was confirmed that the animals recovered from anesthesia under close observation in a temperature-regulated incubator, they were allowed to go back to their cage.
5.3. EMG and VDAT
One day following the third bTBI (i.e., day 8), EMG and VDAT were measured (Fig. 1A) during ankle dorsiflexion in each animal to assess the magnitude of spasticity/rigidity, as previously described (Bose et al., 2002, 2013, 2012; Thompson et al., 1996; Wang et al., 2002). Briefly, awake rats were secured in a custom-designed trunk immobilization device that permitted a normal range of ankle rotation. A series of controlled dorsiflexions (12° each) was produced at 612°, 490°, 408°, 350°, 272°, 204°, 136°, and 49° per second with 3-s intervals using an electromechanical shaker (model 405; Ling Dynamic Systems, Royston Herts, UK). An EMG electrode was inserted into a skin fold above the distal convergence of the two-headed gastrocnemius and soleus muscles (i.e., triceps surae muscle), while a reference electrode was placed into a skin fold on the greater trochanter. Using this experimental set-up, spasticity was assessed by quantifying the lengthening resistance of the triceps surae muscle (i.e., VDAT) as well as raw EMG and root mean square (0.707 DC equivalent of the rectified EMG) of EMG bursts. The data acquisition and analysis were performed using a digital acquisition system with LabVIEW graphic programming (version 8.2; National Instruments, Austin, TX, USA).
5.4. Motor coordination/rotorod balance testing
On day 8, motor coordination/vestibulomotor function of the animals in both groups was evaluated using a rotarod (Columbus Instrument, Columbus, OH, USA). The rotorod we used is featured with variable speed rod rotation in the range of 0 to 99 RPM with an electronic timer and a fall sensor for each animal (“Economex” Columbus Instrument, Columbus, Ohio). Timers are activated by the operator at the moment the animal is placed on the rod and automatically stopped when the animal drops. Stainless steel compartments below the rod restrain the animal from escape once it drops from the rod. The animal’s task was to walk on the rungs as they rotated. The animal was allowed to remain stationary for 10 s at 0 rpm. The rotational speed was slowly increased to 3 rpm for 10 s and was steadily increased (automatic preset) by 3 rpm at 10-s intervals until the maximum rpm of 30 was reached. The animal remained on the device at this speed for another 20 s until the 2–min test period elapsed. The trial ended if the animal fell completely off the rungs or gripped the device and spun around without attempting to walk on the rungs. Animals were tested for three trials per day with at least 10-min interval, and the mean duration on the rotating rod was recorded. There was no pre-training, however, data of the first trial was not recorded. The reliability and sensitivity of these measures were previously reported (Hamm, 2001).
5.5. Tissue dissection
On day 9, animals in both groups were euthanized with Euthasol® (Virbac, Westlake, TX, USA) and the MCx, HPC, VN, LC, and LSC were carefully dissected based on the anatomical maps (Chiasson, 1980; Paxinos and Watson, 2009; Watson et al., 2009). The MCx was collected from the most rostral portion (bregma – 5.64 mm) to the middle of the cerebrum (bregma – 1.20 mm). The HPC was taken out from the most rostral portion (bregma – 1.72 mm) to the midpoint between the dorsal and ventral HPC (bregma – 4.08 mm). As for the LC, the blue tissue (due to melanin granules inside the LC neurons) in the dorsal surface of the pons was carefully collected with forceps (from bregma – 9.48 mm to – 10.20 mm). The VN was dissected from bregma – 10.68 mm to – 12.18 mm. The LSC (2–5) was taken out based on lumbar enlargement.
5.6. HPLC with ECD
Two days after the last bTBI (i.e., day 9), animals were euthanized with Euthasol® to collect MCx, HPC, VN, LC, and LSC. Tissues were immediately snap-frozen in liquid nitrogen and stored at −80 °C until HPLC/ECD analysis was performed. All samples were sonicated in 0.1 M perchloric acid (CAT#: 311421, MilliporeSigma, St. Louis, MO, USA) and then centrifuged at 40,000g for 20 min. Following filtration of supernatants through a 0.2-μm pore, a part of the filtrate was used to determine protein concentration by bicinchoninic acid assay to correct for the variation in sample size. Various concentrations of standard solutions of NE (CAT#: A7257, MilliporeSigma), DA (CAT#: H8502, MilliporeSigma), and 5-HT (CAT#: H9523, MilliporeSigma) were also prepared in 0.1 M perchloric acid. An applied composition of the mobile phase was 0.1 mM ethylenediaminetetraacetic acid disodium salt dihydrate (CAT#: 27285, MilliporeSigma), 100 mM phosphoric acid (CAT#: 438081, MilliporeSigma), 100 mM citric acid monohydrate (CAT#: C1909, MilliporeSigma), pH 6.0, 600 mg/L 1-octanesulfonic acid sodium salt (CAT#: 384771000, ACROS, Geel, Belgium), and 8% (v/v) acetonitrile (CAT#: 26826–0025, ACROS), as previously described with slight modifications (Reinhoud et al., 2013). For separation of sample components, an Acquity UPLC BEH C18 column (Waters, Milford, MA, USA) was used, while the temperature was kept at 42 °C for both separation and detection. Because the sample preparation was very simple and the HPLC/ECD system worked very accurately, external standardization was performed. To determine the quantity of the monoamines in the brain tissue samples, we used a series of different molar concentrations (nanomolars) of commercial analytical grade monoamines (see above) and developed standard curves. Based on the area under a curve of the chromatogram, molar concentrations of monoamines were determined using a UHPLC ALEXYS Neurotransmitter Analyzer containing a DECADE Elite electrochemical detector (Antec Scientific) and a SenCell with GC working electrode set to 460 mV versus Ag/AgCl. The obtained data were then converted to picogram of monoamine per milligram of tissue lysed.
5.7. Statistical analysis
The number of animals and samples was determined based on the power analyses of previous studies assuming detection of a trauma effect producing a significant mean change of one standard deviation from the reference mean (P < 0.05, power of 0.80). Normality tests were performed as parts of the column statistics analyses to confirm normal distribution of the data. Unpaired t-tests were used to analyze between-group differences. All data are presented as mean ± SEM. P values lower than 0.05 are treated as statistically significant. GraphPad Prism 4 software (GraphPad Software, San Diego, CA, USA) was used for data analysis. For VDAT-EMG relationship, a single factor ANOVA was performed.
HIGHLIGHTS.
Monoamine supplies were altered after repetitive blast traumatic brain injury.
Norepinephrine and dopamine were reduced in the vestibular nuclei after injury.
Serotonin was elevated in the motor cortex and spinal cord after injury.
Spasticity and balance deficit were induced after injury.
Acknowledgments
This study was supported by the United States Department of Veterans Affairs Rehabilitation Research and Development Service [Merit Review Award nos. 1I01RX003123–01A1 and 1I01RX001005–01A2]. This work was also partly supported by NIH Translational Outcomes Project in Neurotrauma (TOP-NT) (UG3/UH3) [grant # 1 UG3 NS106938–01]. We thank graphic artist, Emma Nicole Raymaker for her drawings of the brain, spinal cord, and muscle tissues, as well as Corey Astrom, ELS, for her editorial expertise.
Abbreviations:
- TBI
traumatic brain injury
- bTBI
blast-induced TBI
- CST
corticospinal tract
- MCx
motor cortex
- NE
norepinephrine
- DA
dopamine
- 5-HT
serotonin
- VN
vestibular nuclei
- EMG
electromyography
- VDAT
velocity-dependent ankle torque
- HPLC
high-performance liquid chromatography
- ECD
electrochemical detection
- LC
locus coeruleus
- LSC
lumbar spinal cord
- HPC
hippocampus
- NA-R
noradrenergic receptor
- GABA
γ-aminobutyric acid
- LVST
lateral vestibulospinal tract
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Tsuda and Golam have conducted the experiments of HPLC/ECD, while Tsuda wrote the whole manuscript. Hou and Nelson have been responsible for the behavioral experiments. Bernavil and Richardson have significantly contributed to the data analysis of HPLC/ECD. Wang has induced repetitive blast-induced traumatic brain injuries (bTBI) in animals. Thompson and Bose have designed and supervised the whole project and provided necessary scientific and technical advice to authors.
References
- Ahlman H, Grillner S, Udo M, 1971. The effect of 5-HTP on the static fusimotor activity and the tonic stretch reflex of an extensor muscle. Brain Res. 27, 393–396. [DOI] [PubMed] [Google Scholar]
- Andrews C, Knowles L, Hancock J, 1973. Control of the tonic vibration reflex by the brain stem reticular formation in the cat. J. Neurol. Sci. 18, 217–226. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Rosen I, 1972. Functional role of afferent inputs to the monkey motor cortex. Brain Res. 40, 3–5. [DOI] [PubMed] [Google Scholar]
- Awenowicz PW, Porter LL, 2002. Local application of dopamine inhibits pyramidal tract neuron activity in the rodent motor cortex. J. Neurophysiol. 88, 3439–3451. [DOI] [PubMed] [Google Scholar]
- Barresi M, Caldera M, Grasso C, Li Volsi G, Licata F, Santangelo F, 2009. Noradrenergic modulation of neuronal responses to glutamate in the vestibular complex. Neurosci. Lett. 464, 173–178. [DOI] [PubMed] [Google Scholar]
- Barresi M, Grasso C, Licata F, Li Volsi G, 2014. Noradrenergic modulation of neuronal responses to n-methyl-d-aspartate in the vestibular nuclei: an electrophysiological and immunohistochemical study. Neuroscience 265, 172–183. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, De Serres SJ, Stein RB, 1996a. Gain of the triceps surae stretch reflex in decerebrate and spinal cats during postural and locomotor activities. J. Physiol. 496 (Pt 3), 837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, De Serres SJ, Stein RB, 1996b. Regulation of soleus muscle spindle sensitivity in decerebrate and spinal cats during postural and locomotor activities. J. Physiol. 495 (Pt 3), 835–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M, 1998a. Short-term plasticity in hindlimb motoneurons of decerebrate cats. J. Neurophysiol. 80, 2038–2045. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M, 1998b. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J. Neurophysiol. 80, 2023–2037. [DOI] [PubMed] [Google Scholar]
- Borrini L, Bensmail D, Thiebaut JB, Hugeron C, Rech C, Jourdan C, 2014. Occurrence of adverse events in long-term intrathecal baclofen infusion: a 1-year follow-up study of 158 adults. Arch. Phys. Med. Rehabil. 95, 1032–1038. [DOI] [PubMed] [Google Scholar]
- Bos R, Sadlaoud K, Boulenguez P, Buttigieg D, Liabeuf S, Brocard C, Haase G, Bras H, Vinay L, 2013. Activation of 5-HT2A receptors upregulates the function of the neuronal K-Cl cotransporter KCC2. Proc. Natl. Acad. Sci. U.S.A. 110, 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose P, Parmer R, Thompson FJ, 2002. Velocity-dependent ankle torque in rats after contusion injury of the midthoracic spinal cord: time course. J. Neurotrauma 19, 1231–1249. [DOI] [PubMed] [Google Scholar]
- Bose P, Hou J, Nelson R, Nissim N, Parmer R, Keener J, Wacnik PW, Thompson FJ, 2013. Effects of acute intrathecal baclofen in an animal model of TBI-induced spasticity, cognitive, and balance disabilities. J. Neurotrauma 30, 1177–1191. [DOI] [PubMed] [Google Scholar]
- Bose P, W.D.C., Parmer R, Wiley RG, Thompson FJ, 2001. Monoamine modulation of spinal reflex excitability of the lower limb in the rat: Intrathecal infusion of Anti-DBH saporin toxin – time course for behavior neuroscience. In Society for Neuroscience. Vol. Vol. 771.3. ed.êds., San Diego, CA. [Google Scholar]
- Bose PK, Hou J, Parmer R, Reier PJ, Thompson FJ, 2012. Altered patterns of reflex excitability, balance, and locomotion following spinal cord injury and locomotor training. Front. Physiol. 3, 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botte MJ, Nickel VL, Akeson WH, 1988. Spasticity and contracture. Physiologic aspects of formation. Clin. Orthop. Relat. Res. 7–18. [PubMed] [Google Scholar]
- Brown AR, Teskey GC, 2014. Motor cortex is functionally organized as a set of spatially distinct representations for complex movements. J. Neurosci. 34, 13574–13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busto R, Dietrich WD, Globus MY, Alonso O, Ginsberg MD, 1997. Extracellular release of serotonin following fluid-percussion brain injury in rats. J. Neurotrauma 14, 35–42. [DOI] [PubMed] [Google Scholar]
- Calabro RS, D’Aleo G, Sessa E, Leo A, De Cola MC, Bramanti P, 2014. Sexual dysfunction induced by intrathecal baclofen administration: is this the price to pay for severe spasticity management? J. Sex Med. 11, 1807–1815. [DOI] [PubMed] [Google Scholar]
- Cardoso AL, Quintaneiro C, Seabra H, Teixeira C, 2014. Cardiac arrest due to baclofen withdrawal syndrome. BMJ Case Rep. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Artigas F, 2013. Serotonin modulation of cortical neurons and networks. Front. Integr. Neurosci. 7, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Huang EY, Kuo TT, Ma HI, Hoffer BJ, Tsui PF, Tsai JJ, Chou YC, Chiang YH, 2015. Dopamine release impairment in striatum after different levels of cerebral cortical fluid percussion injury. Cell Transplant. 24, 2113–2128. [DOI] [PubMed] [Google Scholar]
- Chiasson R, 1980. Laboratory anatomy of the white rat. Vol., Wm. C. Brown Company Publishers, United States. [Google Scholar]
- Corneil BD, Camp AJ, 2018. Animal models of vestibular evoked myogenic potentials: the past, present, and future. Front. Neurol. 9, 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico JM, Li Y, Bennett DJ, Gorassini MA, 2013. Reduction of spinal sensory transmission by facilitation of 5-HT1B/D receptors in noninjured and spinal cord-injured humans. J. Neurophysiol. 109, 1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentel C, Palamiuc L, Henriques A, Lannes B, Spreux-Varoquaux O, Gutknecht L, Rene F, Echaniz-Laguna A, Gonzalez de Aguilar JL, Lesch KP, Meininger V, Loeffler JP, Dupuis L, 2013. Degeneration of serotonergic neurons in amyotrophic lateral sclerosis: a link to spasticity. Brain. 136, 483–493. [DOI] [PubMed] [Google Scholar]
- Di Mauro M, Bronzi D, Li Volsi G, Licata F, Lombardo P, Santangelo F, 2008. Noradrenaline modulates neuronal responses to GABA in vestibular nuclei. Neuroscience 153, 1320–1331. [DOI] [PubMed] [Google Scholar]
- Ebbesen CL, Brecht M, 2017. Motor cortex – to act or not to act? Nat. Rev. Neurosci. 18, 694–705. [DOI] [PubMed] [Google Scholar]
- Ebbesen CL, Insanally MN, Kopec CD, Murakami M, Saiki A, Erlich JC, 2018. More than just a “motor”: recent surprises from the frontal cortex. J. Neurosci. 38, 9402–9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH, Trott JR, 1975. Proceedings: Facilitation of the tonic vibration reflex in the spinal cat by 5-hydroxytryptophan (5-HTP). J. Physiol. 249, 54P–56P. [PubMed] [Google Scholar]
- Ellaway PH, Taylor A, Durbaba R, 2015. Muscle spindle and fusimotor activity in locomotion. J. Anat. 227, 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausti SA, Wilmington DJ, Gallun FJ, Myers PJ, Henry JA, 2009. Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. J. Rehabil. Res. Dev. 46, 797–810. [DOI] [PubMed] [Google Scholar]
- Francois B, Vacher P, Roustan J, Salle JY, Vidal J, Moreau JJ, Vignon P, 2001. Intrathecal baclofen after traumatic brain injury: early treatment using a new technique to prevent spasticity. J. Trauma 50, 158–161. [DOI] [PubMed] [Google Scholar]
- Garcia-Ramirez DL, Calvo JR, Hochman S, Quevedo JN, 2014. Serotonin, dopamine and noradrenaline adjust actions of myelinated afferents via modulation of presynaptic inhibition in the mouse spinal cord. PLoS ONE 9, e89999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracies JM, Brashear A, Jech R, McAllister P, Banach M, Valkovic P, Walker H, Marciniak C, Deltombe T, Skoromets A, Khatkova S, Edgley S, Gul F, Catus F, De Fer BB, Vilain C, Picaut P, International Abobotulinumtoxin AAULSSG, 2015. Safety and efficacy of abobotulinumtoxinA for hemiparesis in adults with upper limb spasticity after stroke or traumatic brain injury: a double-blind randomised controlled trial. Lancet Neurol. 14, 992–1001. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, 2001. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J. Neurotrauma 18, 1207–1216. [DOI] [PubMed] [Google Scholar]
- Heckmann CJ, Gorassini MA, Bennett DJ, 2005. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31, 135–156. [DOI] [PubMed] [Google Scholar]
- Herbin M, Simonis C, Reveret L, Hackert R, Libourel PA, Eugene D, Diaz J, de Waele C, Vidal PP, 2016. Dopamine modulates motor control in a specific plane related to support. PLoS ONE 11, e0155058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer ME, Balaban C, Gottshall K, Balough BJ, Maddox MR, Penta JR, 2010. Blast exposure: vestibular consequences and associated characteristics. Otol. Neurotol. 31, 232–236. [DOI] [PubMed] [Google Scholar]
- Hornung JP, 2003. The human raphe nuclei and the serotonergic system. J. Chem. Neuroanat. 26, 331–343. [DOI] [PubMed] [Google Scholar]
- Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR, 2011. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J. Neurosci. 31, 2481–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosp JA, Luft AR, 2013. Dopaminergic meso-cortical projections to m1: role in motor learning and motor cortex plasticity. Front. Neurol. 4, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Nelson R, Nissim N, Parmer R, Thompson FJ, Bose P, 2014. Effect of combined treadmill training and magnetic stimulation on spasticity and gait impairments after cervical spinal cord injury. J. Neurotrauma 31, 1088–1106. [DOI] [PubMed] [Google Scholar]
- Jang SH, Park SM, Kim SH, Ahn SH, Cho YW, Ahn MO, 2004. The effect of selective tibial neurotomy and rehabilitation in a quadriplegic patient with ankle spasticity following traumatic brain injury. Yonsei Med. J. 45, 743–747. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Gladden MH, Czarkowska-Bauch J, 1998. Modulation of responses of feline gamma-motoneurones by noradrenaline, tizanidine and clonidine. J. Physiol. 512 (Pt 2), 521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HJ, Han SH, Min BI, Cho YW, 2001. 5-HT1A receptor-mediated activation of G-protein-gated inwardly rectifying K+ current in rat periaqueductal gray neurons. Neuropharmacology 41, 175–185. [DOI] [PubMed] [Google Scholar]
- Kawa L, Arborelius UP, Yoshitake T, Kehr J, Hokfelt T, Risling M, Agoston D, 2015. Neurotransmitter systems in a mild blast traumatic brain injury model: catecholamines and serotonin. J. Neurotrauma 32, 1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa L, Kamnaksh A, Long JB, Arborelius UP, Hokfelt T, Agoston DV, Risling M, 2018. A comparative study of two blast-induced traumatic brain injury models: changes in monoamine and galanin systems following single and repeated exposure. Front. Neurol. 9, 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AE, Yu J, Horvath E, Marion DW, Dixon CE, 2001. The selective 5-HT(1A) receptor agonist repinotan HCl attenuates histopathology and spatial learning deficits following traumatic brain injury in rats. Neuroscience 106, 547–555. [DOI] [PubMed] [Google Scholar]
- Kline AE, Massucci JL, Dixon CE, Zafonte RD, Bolinger BD, 2004. The therapeutic efficacy conferred by the 5-HT(1A) receptor agonist 8-Hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) after experimental traumatic brain injury is not mediated by concomitant hypothermia. J. Neurotrauma 21, 175–185. [DOI] [PubMed] [Google Scholar]
- Kline AE, McAloon RL, Henderson KA, Bansal UK, Ganti BM, Ahmed RH, Gibbs RB, Sozda CN, 2010. Evaluation of a combined therapeutic regimen of 8-OH-DPAT and environmental enrichment after experimental traumatic brain injury. J. Neurotrauma 27, 2021–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann O, Jeltsch H, Lehnardt O, Pain L, Lazarus C, Cassel JC, 2000. Combined lesions of cholinergic and serotonergic neurons in the rat brain using 192 IgG-saporin and 5,7-dihydroxytryptamine: neurochemical and behavioural characterization. Eur. J. Neurosci. 12, 67–79. [DOI] [PubMed] [Google Scholar]
- Licata F, Li Volsi G, Maugeri G, Ciranna L, Santangelo F, 1993. Effects of noradrenaline on the firing rate of vestibular neurons. Neuroscience 53, 149–158. [DOI] [PubMed] [Google Scholar]
- Liposits Z, Phelix C, Paull WK, 1986. Adrenergic innervation of corticotropin releasing factor (CRF)-synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A combined light and electron microscopic immunocytochemical study. Histochemistry 84, 201–205. [DOI] [PubMed] [Google Scholar]
- Liu M, Bachstetter AD, Cass WA, Lifshitz J, Bing G, 2017. Pioglitazone attenuates neuroinflammation and promotes dopaminergic neuronal survival in the nigrostriatal system of rats after diffuse brain injury. J. Neurotrauma 34, 414–422. [DOI] [PubMed] [Google Scholar]
- Llado-Pelfort L, Assie MB, Newman-Tancredi A, Artigas F, Celada P, 2010. Preferential in vivo action of F15599, a novel 5-HT(1A) receptor agonist, at postsynaptic 5-HT(1A) receptors. Br. J. Pharmacol. 160, 1929–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentzen J, Nielsen D, Holm K, Baagoe S, Grey MJ, Nielsen JB, 2012. Neural tension technique is no different from random passive movements in reducing spasticity in patients with traumatic brain injury. Disabil. Rehabil. 34, 1978–1985. [DOI] [PubMed] [Google Scholar]
- Lucetti C, Del Dotto P, Gambaccini G, Ceravolo R, Logi C, Berti C, Rossi G, Bianchi MC, Tosetti M, Murri L, Bonuccelli U, 2007. Influences of dopaminergic treatment on motor cortex in Parkinson disease: a MRI/MRS study. Mov. Disord. 22, 2170–2175. [DOI] [PubMed] [Google Scholar]
- Mas MF, DiTommaso C, Li S, 2018. Phenol neurolysis for the management of shoulder spasticity in early recovery from traumatic brain injury-a case report. PM R. [DOI] [PubMed] [Google Scholar]
- McCall AA, Miller DM, Yates BJ, 2017. Descending influences on vestibulospinal and vestibulosympathetic reflexes. Front. Neurol. 8, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh TK, Yu T, Gennarelli TA, 1994. Alterations in regional brain catecholamine concentrations after experimental brain injury in the rat. J. Neurochem. 63, 1426–1433. [DOI] [PubMed] [Google Scholar]
- Mendell LM, 1984. Modifiability of spinal synapses. Physiol. Rev. 64, 260–324. [DOI] [PubMed] [Google Scholar]
- Miller DM, Klein CS, Suresh NL, Rymer WZ, 2014. Asymmetries in vestibular evoked myogenic potentials in chronic stroke survivors with spastic hypertonia: evidence for a vestibulospinal role. Clin. Neurophysiol. 125, 2070–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Tabira T, 2002a. Chronic stress impairs rotarod performance in rats: implications for depressive state. Pharmacol. Biochem. Behav. 71, 79–84. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Nagata M, Ishige A, Sasaki H, Tabira T, 2002b. Dopamine-receptor stimulation in the prefrontal cortex ameliorates stress-induced rotarod impairment. Pharmacol. Biochem. Behav. 72, 723–728. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Aburada M, Tabira T, 2003. Saiko-ka-ryukotsu-borei-to, a herbal medicine, ameliorates chronic stress-induced depressive state in rotarod performance. Pharmacol. Biochem. Behav. 75, 419–425. [DOI] [PubMed] [Google Scholar]
- Monaco CM, Gebhardt KM, Chlebowski SM, Shaw KE, Cheng JP, Henchir JJ, Zupa MF, Kline AE, 2014. A combined therapeutic regimen of buspirone and environmental enrichment is more efficacious than either alone in enhancing spatial learning in brain-injured pediatric rats. J. Neurotrauma 31, 1934–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta F, Antonello CE, 2014. Analysis of complications in 430 consecutive pediatric patients treated with intrathecal baclofen therapy: 14-year experience. J Neurosurg Pediatr. 13, 301–306. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Chakravarty A, 2010. Spasticity mechanisms - for the clinician. Front. Neurol. 1, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa G, Hou J, Tsuda S, Nelson R, Sinharoy A, Wilkie Z, Pandey R, Caudle RM, Neubert JK, Thompson FJ, Bose P, 2016. Trigeminal neuroplasticity underlies allodynia in a preclinical model of mild closed head traumatic brain injury (cTBI). Neuropharmacology 107, 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase-Richardson R, McNamee S, Howe LL, Massengale J, Peterson M, Barnett SD, Harris O, McCarthy M, Tran J, Scott S, Cifu DX, 2013. Descriptive characteristics and rehabilitation outcomes in active duty military personnel and veterans with disorders of consciousness with combat- and noncombat-related brain injury. Arch. Phys. Med. Rehabil. 94, 1861–1869. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR, 1986. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 396, 77–96. [DOI] [PubMed] [Google Scholar]
- Olsen AS, Sozda CN, Cheng JP, Hoffman AN, Kline AE, 2012. Traumatic brain injury-induced cognitive and histological deficits are attenuated by delayed and chronic treatment with the 5-HT1A-receptor agonist buspirone. J. Neurotrauma 29, 1898–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordia JI, Fischer E, Adamski E, Spatz EL, 2002. Continuous intrathecal baclofen infusion delivered by a programmable pump for the treatment of severe spasticity following traumatic brain injury. Neuromodulation 5, 103–107. [DOI] [PubMed] [Google Scholar]
- Pasquina P, Kirtley R, Ling G, 2014. Moderate-to-severe traumatic brain injury. Semin. Neurol. 34, 572–583. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2009. The rat brain in stereotaxic coordinates. Vol., Elsevier, Amsterdam. [DOI] [PubMed] [Google Scholar]
- Peng SY, Zhuang QX, Zhang YX, Zhang XY, Wang JJ, Zhu JN, 2016. Excitatory effect of norepinephrine on neurons in the inferior vestibular nucleus and the underlying receptor mechanism. J. Neurosci. Res. 94, 736–748. [DOI] [PubMed] [Google Scholar]
- Perez-Arredondo A, Cazares-Ramirez E, Carrillo-Mora P, Martinez-Vargas M, Cardenas-Rodriguez N, Coballase-Urrutia E, Alemon-Medina R, Sampieri A 3rd, Navarro L, Carmona-Aparicio L, 2016. Baclofen in the therapeutic of sequele of traumatic brain injury: spasticity. Clin. Neuropharmacol. 39, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Rasmussen HB, Christensen RK, Petersen AV, 2013. Modulation of the intrinsic properties of motoneurons by serotonin. Curr. Pharm. Des. 19, 4371–4384. [DOI] [PubMed] [Google Scholar]
- Phelps TI, Bondi CO, Mattiola VV, Kline AE, 2017. Relative to typical antipsychotic drugs, aripiprazole is a safer alternative for alleviating behavioral disturbances after experimental brain trauma. Neurorehabil. Neural Repair 31, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Cunningham ET Jr., Widmaier EP, 1989. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocr. Rev. 10, 437–458. [DOI] [PubMed] [Google Scholar]
- Podda MV, Johnston AR, Tolu E, Dutia MB, 2001. Modulation of rat medial vestibular nucleus neurone activity by vasopressin and noradrenaline in vitro. Neurosci. Lett. 298, 91–94. [DOI] [PubMed] [Google Scholar]
- Pompeiano O, 1991. The role of different size vestibulospinal neurons in the static control of posture. Arch. Ital. Biol. 129, 21–41. [PubMed] [Google Scholar]
- Pompeiano O, Horn E, d’Ascanio P, 1991. Locus coeruleus and dorsal pontine reticular influences on the gain of vestibulospinal reflexes. Prog. Brain Res. 88, 435–462. [DOI] [PubMed] [Google Scholar]
- Potter-Nerger M, Reich MM, Colebatch JG, Deuschl G, Volkmann J, 2012. Differential effect of dopa and subthalamic stimulation on vestibular activity in Parkinson’s disease. Mov. Disord. 27, 1268–1275. [DOI] [PubMed] [Google Scholar]
- Puig MV, Watakabe A, Ushimaru M, Yamamori T, Kawaguchi Y, 2010. Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J. Neurosci. 30, 2211–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhoud NJ, Brouwer HJ, van Heerwaarden LM, Korte-Bouws GA, 2013. Analysis of glutamate, GABA, noradrenaline, dopamine, serotonin, and metabolites using microbore UHPLC with electrochemical detection. ACS Chem. Neurosci. 4, 888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi L, Edwards MJ, Fasano A, Bhatia KP, Stamelou M, 2018. Progressive spasticity, supranuclear gaze palsy and postural instability, without parkinsonism: what’s in a phenotype? J. Neurol. Sci. 390, 84–86. [DOI] [PubMed] [Google Scholar]
- Rifici C, Kofler M, Kronenberg M, Kofler A, Bramanti P, Saltuari L, 1994. Intrathecal baclofen application in patients with supraspinal spasticity secondary to severe traumatic brain injury. Funct. Neurol. 9, 29–34. [PubMed] [Google Scholar]
- Russell AL, Handa RJ, Wu TJ, 2018. Sex-dependent effects of mild blast-induced traumatic brain injury on corticotropin-releasing factor receptor gene expression: potential link to anxiety-like behaviors. Neuroscience 392, 1–12. [DOI] [PubMed] [Google Scholar]
- Sajja VS, Galloway M, Ghoddoussi F, Kepsel A, VandeVord P, 2013. Effects of blast-induced neurotrauma on the nucleus accumbens. J. Neurosci. Res. 91, 593–601. [DOI] [PubMed] [Google Scholar]
- Scherer MR, Schubert MC, 2009. Traumatic brain injury and vestibular pathology as a comorbidity after blast exposure. Phys. Ther. 89, 980–992. [DOI] [PubMed] [Google Scholar]
- Schiemann J, Puggioni P, Dacre J, Pelko M, Domanski A, van Rossum MC, Duguid I, 2015. Cellular mechanisms underlying behavioral state-dependent bidirectional modulation of motor cortex output. Cell Rep. 11, 1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AG, Meabon JS, Pagulayan KF, Hendrickson RC, Meeker KD, Cline M, Li G, Sikkema C, Wilkinson CW, Perl DP, Raskind MR, Peskind ER, Clark JJ, Cook DG, 2017. Blast-related disinhibition and risk seeking in mice and combat Veterans: Potential role for dysfunctional phasic dopamine release. Neurobiol. Dis. 106, 23–34. [DOI] [PubMed] [Google Scholar]
- Schmit BD, Rymer WZ, 2001. Identification of static and dynamic components of reflex sensitivity in spastic elbow flexors using a muscle activation model. Ann. Biomed. Eng. 29, 330–339. [DOI] [PubMed] [Google Scholar]
- Schuerger RJ, Balaban CD, 1993. Immunohistochemical demonstration of regionally selective projections from locus coeruleus to the vestibular nuclei in rats. Exp. Brain Res. 92, 351–359. [DOI] [PubMed] [Google Scholar]
- Schuerger RJ, Balaban CD, 1999. Organization of the coeruleo-vestibular pathway in rats, rabbits, and monkeys. Brain Res. Brain Res. Rev. 30, 189–217. [DOI] [PubMed] [Google Scholar]
- Shin SS, Bray ER, Zhang CQ, Dixon CE, 2011. Traumatic brain injury reduces striatal tyrosine hydroxylase activity and potassium-evoked dopamine release in rats. Brain Res. 1369, 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively SB, Perl DP, 2012. Traumatic brain injury, shell shock, and posttraumatic stress disorder in the military–past, present, and future. J Head Trauma Rehabil 27, 234–239. [DOI] [PubMed] [Google Scholar]
- Taira T, Ueta T, Katayama Y, Kimizuka M, Nemoto A, Mizusawa H, Liu M, Koito M, Hiro Y, Tanabe H, 2013. Rate of complications among the recipients of intrathecal baclofen pump in Japan: a multicenter study. Neuromodulation 16, 266–272 discussion 272. [DOI] [PubMed] [Google Scholar]
- Tanaka YH, Tanaka YR, Fujiyama F, Furuta T, Yanagawa Y, Kaneko T, 2011. Local connections of layer 5 GABAergic interneurons to corticospinal neurons. Front. Neural Circuits 5, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson FJ, Fernandex JJ, 1975. Patterns of cortical projection to hindlimb muscle motoneurone pools. Brain Res. 97, 33–46. [DOI] [PubMed] [Google Scholar]
- Thompson FJ, Reier PJ, Lucas CC, Parmer R, 1992. Altered patterns of reflex excitability subsequent to contusion injury of the rat spinal cord. J. Neurophysiol. 68, 1473–1486. [DOI] [PubMed] [Google Scholar]
- Thompson FJ, Reier PJ, Parmer R, Lucas CC, 1993. Inhibitory control of reflex excitability following contusion injury and neural tissue transplantation. Adv. Neurol. 59, 175–184. [PubMed] [Google Scholar]
- Thompson FJ, Browd CR, Carvalho PM, Hsiao J, 1996. Velocity-dependent ankle torque in the normal rat. NeuroReport 7, 2273–2276. [PubMed] [Google Scholar]
- Thompson FJ, Parmer R, Reier PJ, 1998. Alteration in rate modulation of reflexes to lumbar motoneurons after midthoracic spinal cord injury in the rat. I. Contusion injury. J Neurotrauma 15, 495–508. [DOI] [PubMed] [Google Scholar]
- Thompson FJ, Parmer R, Reier PJ, Wang DC, Bose P, 2001a. Scientific basis of spasticity: insights from a laboratory model. J. Child Neurol. 16, 2–9. [DOI] [PubMed] [Google Scholar]
- Thompson FJ, Reier PJ, Uthman B, Mott S, Fessler RG, Behrman A, Trimble M, Anderson DK, Wirth ED 3rd, 2001b. Neurophysiological assessment of the feasibility and safety of neural tissue transplantation in patients with syringomyelia. J. Neurotrauma 18, 931–945. [DOI] [PubMed] [Google Scholar]
- Toklu HZ, Yang Z, Oktay S, Sakarya Y, Kirichenko N, Matheny MK, Muller-Delp J, Strang K, Scarpace PJ, Wang KKW, Tumer N, 2018. Overpressure blast injury-induced oxidative stress and neuroinflammation response in rat frontal cortex and cerebellum. Behav. Brain Res. 340, 14–22. [DOI] [PubMed] [Google Scholar]
- Trimble MH, Behrman AL, Flynn SM, Thigpen MT, Thompson FJ, 2001. Acute effects of locomotor training on overground walking speed and H-reflex modulation in individuals with incomplete spinal cord injury. J. Spinal Cord Med. 24, 74–80. [DOI] [PubMed] [Google Scholar]
- Tumer N, Svetlov S, Whidden M, Kirichenko N, Prima V, Erdos B, Sherman A, Kobeissy F, Yezierski R, Scarpace PJ, Vierck C, Wang KK, 2013. Overpressure blast-wave induced brain injury elevates oxidative stress in the hypothalamus and catecholamine biosynthesis in the rat adrenal medulla. Neurosci. Lett. 544, 62–67. [DOI] [PubMed] [Google Scholar]
- van Bregt DR, Thomas TC, Hinzman JM, Cao T, Liu M, Bing G, Gerhardt GA, Pauly JR, Lifshitz J, 2012. Substantia nigra vulnerability after a single moderate diffuse brain injury in the rat. Exp. Neurol. 234, 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitrac C, Benoit-Marand M, 2017. Monoaminergic modulation of motor cortex function. Front. Neural Circuits 11, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, Sokoloski JE, Ren D, Chen X, Khan AS, Zafonte RD, Michael AC, Dixon CE, 2005. Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J. Neurochem. 95, 457–465. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Drewencki LL, Chen X, Santos FR, Khan AS, Harun R, Torres GE, Michael AC, Dixon CE, 2009. Chronic methylphenidate treatment enhances striatal dopamine neurotransmission after experimental traumatic brain injury. J. Neurochem. 108, 986–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DC, Bose P, Parmer R, Thompson FJ, 2002. Chronic intrathecal baclofen treatment and withdrawal: I. Changes in ankle torque and hind limb posture in normal rats. J. Neurotrauma 19, 875–886. [DOI] [PubMed] [Google Scholar]
- Watson C, George P, Gulgun K, 2009. The Spinal Cord: A Christopher and Dana Reeve Foundation Text and Atlas. Academic Press, Amsterdam. [Google Scholar]
- Wei K, Glaser JI, Deng L, Thompson CK, Stevenson IH, Wang Q, Hornby TG, Heckman CJ, Kording KP, 2014. Serotonin affects movement gain control in the spinal cord. J. Neurosci. 34, 12690–12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Cao S, Chao H, Liu Y, Ji J, 2016. Sex-related differences in striatal dopaminergic system after traumatic brain injury. Brain Res. Bull. 124, 214–221. [DOI] [PubMed] [Google Scholar]
- Yablon SA, Agana BT, Ivanhoe CB, Boake C, 1996. Botulinum toxin in severe upper extremity spasticity among patients with traumatic brain injury: an open-labeled trial. Neurology 47, 939–944. [DOI] [PubMed] [Google Scholar]
- Zhai F, Shi F, Wang J, Dai CF, Fan C, 2019. Preliminary study on the mechanism underlying the interaction of balance dysfunction and anxiety disorder. NeuroReport 30, 53–59. [DOI] [PubMed] [Google Scholar]
- Zong H, Ma F, Zhang L, Lu H, Gong J, Cai M, Lin H, Zhu Y, Hou C, 2016. Hindlimb spasticity after unilateral motor cortex lesion in rats is reduced by contralateral nerve root transfer. Biosci. Rep. 36. [DOI] [PMC free article] [PubMed] [Google Scholar]


