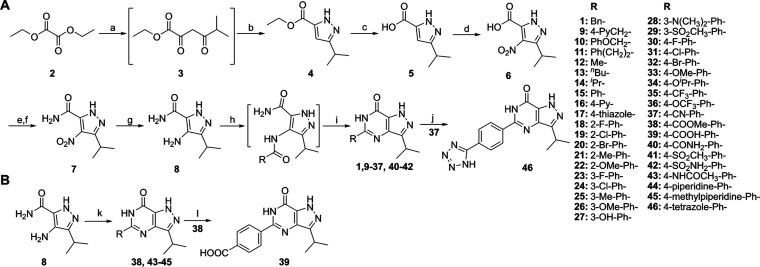
Scheme 1. Synthesis of Pyrazolopyrimidinone Analogs.
Reagents and conditions: (a) 3-methylbutan-2-one, NaOEt, EtOH, 60 °C, 2 h; (b) N2H4·H2O, EtOH, reflux, 2 h, 54% over two steps; (c) NaOH, 1,4-dioxane/H2O 1:1 (v/v), 50 °C, 3 h, 62%; (d) conc. H2SO4, 65% HNO3, 60 °C, 3 h, 64%; (e) cat. DMF, (COCl)2, DCM, 0 °C, 1 h, then RT, 2 h; (f) 7 M NH3 in MeOH, 0 °C, 30 min, 69% over two steps; (g) 10% Pd/C, H2 (g), EtOH, 60 °C, 18 h, 93%; (h) RCOOH, TEA, bromo-tris-pyrrolidino-phosphonium hexafluorophosphate (PyBroP), DCE, microwave irradiation (MW) 120 °C, 20 min; (i) KOtBu, iPrOH, MW 130 °C, 30 min, 4–90% over two steps; (j) NaN3, NH4Cl, DMF, MW 160 °C, 2 h, 69%; (k) RCHO, I2, DMF, 80 °C, 16 h, 23–47%; (l) LiOH, 1,4-dioxane/H2O 1:1 (v/v), reflux, 2 h, 75%.