Abstract
BACKGROUND:
Aortic pulse wave velocity (PWV) predicts cardiovascular events (CVEs) and total mortality (TM), but previous studies proposing actionable PWV thresholds have limited generalizability. This individual-participant meta-analysis is aimed at defining, testing calibration, and validating an outcome-driven threshold for PWV, using 2 populations studies, respectively, for derivation IDCARS (International Database of Central Arterial Properties for Risk Stratification) and replication MONICA (Monitoring of Trends and Determinants in Cardiovascular Disease Health Survey – Copenhagen).
METHODS:
A risk-carrying PWV threshold for CVE and TM was defined by multivariable Cox regression, using stepwise increasing PWV thresholds and by determining the threshold yielding a 5-year risk equivalent with systolic blood pressure of 140 mm Hg. The predictive performance of the PWV threshold was assessed by computing the integrated discrimination improvement and the net reclassification improvement.
RESULTS:
In well-calibrated models in IDCARS, the risk-carrying PWV thresholds converged at 9 m/s (10 m/s considering the anatomic pulse wave travel distance). With full adjustments applied, the threshold predicted CVE (hazard ratio [CI]: 1.68 [1.15–2.45]) and TM (1.61 [1.01–2.55]) in IDCARS and in MONICA (1.40 [1.09–1.79] and 1.55 [1.23–1.95]). In IDCARS and MONICA, the predictive accuracy of the threshold for both end points was ≈0.75. Integrated discrimination improvement was significant for TM in IDCARS and for both TM and CVE in MONICA, whereas net reclassification improvement was not for any outcome.
CONCLUSIONS:
PWV integrates multiple risk factors into a single variable and might replace a large panel of traditional risk factors. Exceeding the outcome-driven PWV threshold should motivate clinicians to stringent management of risk factors, in particular hypertension, which over a person’s lifetime causes stiffening of the elastic arteries as waypoint to CVE and death.
Keywords: cardiovascular diseases, diabetes mellitus, hypertension, pulse wave analysis, metabolic syndrome
NOVELTY AND RELEVANCE.
What Is New?
IDCARS (International Database of Central Arterial Properties for Risk Stratification; N=3378) and MONICA (Monitoring of Trends and Determinants in Cardiovascular Disease Health Survey – Copenhagen; N=2458) are large population studies assuring generalizability.
In well-calibrated multivariable models, the risk-carrying pulse wave velocity (PVW) thresholds in IDCARS converged to 9 m/s, of which the prognostic utility was replicated in MONICA.
The 9-m/s PWV refined risk stratification on top of classical risk factors, albeit to a minor extent.
What Is Relevant?
Although PWV is continuously distributed, clinicians need operational thresholds to base their clinical decisions.
The 9-m/s outcome-driven threshold corresponds to the 10-m/s actionable limit proposed by the European guidelines with correction of the anatomic travel path.
Clinical/Pathophysiological Implications?
Over a person’s lifetime, hypertension leads to irreparable elastin fragmentation in the wall of elastic arteries, thereby causing major premature death and cardiovascular complications. PWV integrates all unmodifiable and modifiable risk factors in a single variable, so that its measurement should be encouraged for risk stratification. Exceeding the threshold should motivate clinicians to stringent management of risk factors, in particular hypertension.
Over the human lifespan, aging and age-related risk factors, such as hypertension and type-2 diabetes, lead to stiffening of the central elastic arteries. Consequently, the systolic load on the arterial walls is cushioned less, a phenomenon further amplified by the early return of reflected waves in late systole, while the tensile force maintaining a continuous blood flow during diastole diminishes.1 Aortic pulse wave velocity (PWV) is the gold standard for the noninvasive assessment of central arterial stiffness2 and predicts adverse cardiovascular outcomes in a continuous manner.1 However, in support of clinical decision-making European3 and Chinese4 guidelines proposed a fixed risk threshold of >12 m/s, which European experts subsequently lowered to >10 m/s, considering the difference between the measured and anatomic pulse wave travel distance.2,5–7
The literature supporting the guideline-endorsed PWV thresholds2–5 consists of patient8–15 and community-based16–19 studies with total or cardiovascular mortality or a composite cardiovascular end point as outcome. In patients with end-stage kidney disease,9–12,15 metabolic syndrome,14 hypertension,8 or in patients undergoing transluminal aortic valve replacement,13 PWV risk thresholds ranged from 10.5 m/s11 to 11.8 m/s.12 In Japanese-Americans,16 Japanese men,20 or middle-aged or elderly community-dwelling individuals,17,19 the PWV risk thresholds ranged from 9.0 m/s18 to 13.7 m/s.19 In a cross-sectional meta-analysis of 16 867 individuals,7 the distribution of PWV was described as function of age in various patient strata, including a subset of 1455 patients with optimal or normal blood pressure (BP) considered to mirror population-based levels. Notwithstanding the merits of the previous publications,11–14,16–19 PWV thresholds derived in patients with advanced disease11–13 or disturbed metabolic profile14 in single-center population cohorts,16–19 in the elderly,17 or based on the PWV distribution rather than adverse health outcomes7 cannot be straightforwardly extrapolated to clinical practice. In view of these potential limitations, the current individual-participant level meta-analysis, covering a wide age range, was prospectively and specifically designed to define, test the calibration, and validate an outcome-driven threshold for PWV, using the IDCARS (International Database of Central Arterial Properties for Risk Stratification)21 as derivation dataset and MONICA study (Monitoring of Trends and Determinants in Cardiovascular Disease Health Survey – Copenhagen) for replication.22
METHODS
Data Availability
All available data are shown within the article and the online-only Supplemental Material. Anonymized data are available from the corresponding author upon request; on condition that an analysis plan is accompanying the request and that the principal investigators of all cohorts approve data sharing.
Study Cohorts
The population studies included in the current meta-analysis met the principles outlined in the Helsinki declaration for investigation of human participants.23 The IDCARS study protocols and the secondary analyses of anonymized data were approved by the competent local Institutional or National Review Boards. Anonymized data from the Copenhagen subset of the MONICA study were used for analysis. Participants gave written informed consent at recruitment and renewed consent at each follow-up visit. The online only Supplemental Material provides full details on the selection of the study population and the methods applied for collecting the clinical, biochemical, and hemodynamic measurements and the statistical analysis.
IDCARS: Derivation Cohort
IDCARS cohorts qualified for inclusion in the present analysis, if peripheral and central BP and cardiovascular risk factors had been measured at baseline, and if follow-up included both fatal and nonfatal outcomes. Eight cohorts met these eligibility criteria (Table S1). Initial enrollment took place from 1985 to 2015. For the present analysis, baseline refers to the first measurement of central and peripheral BP along with cardiovascular risk factors (October 2000 to February 2016). Across studies, the last follow-up took place from October 2012 to December 2018 (Table S1). In the 8 qualifying IDCARS cohorts, 6546 individuals took part in a re-examination including also the vascular examination. Of those, 2706 (41.3%) only underwent a tonometric pulse wave analysis or had a substandard assessment of PWV. Of the remaining 3840 participants, 462 (12.0%) were discarded, because they were aged <30 years, leaving 3378 IDCARS participants for statistical analysis.
MONICA: Replication Cohort
In 1982 to 1984, a random sample of the residents of Glostrup County, one of the Western suburbs of Copenhagen, Denmark, was drawn with the goal to recruit an equal number of women and men aged 30, 40, 50, and 60 years. In 1993 to 1994, the 3785 former participants were invited for a follow-up examination at the Research Center for Prevention and Health in Glostrup, of whom 2493 (65.9%) without history of cardiovascular disease between recruitment and follow-up were examined.22 For the current analysis, 35 (1.40%) were excluded because of inaccurate or missing PWV measurements, leaving 2458 participants for analysis.
BP and PWV Measurement
In IDCARS, brachial BP was the average of the first 2 consecutive readings. Mean arterial pressure (MAP) was peripheral diastolic BP plus one-third of pulse pressure. In all IDCARS cohorts included in the current analysis, PWV was measured by sequential electrocardiographically gated recordings of the arterial pressure waveform at the carotid and femoral arteries. The observers measured the distance from the suprasternal notch to the carotid sampling site (distance A), and from the suprasternal notch to the femoral sampling site (distance B). Pulse wave travel distance was calculated as distance B minus distance A.21 Pulse transit time was the average of 10 consecutive beats.21 PWV is the ratio of the travel distance in meters to transit time in seconds. PWV measurements were discarded if the SEM of 10 beats was >10%.21
In MONICA, a trained nurse obtained 2 consecutive BP readings with a random zero mercury sphygmomanometer, which were averaged for analysis. Immediately thereafter, the trained nurse used 2 piezoelectric pressure transducers (Hellige GmbH, Freiburg im Breisgau, Germany) to record the arterial wave simultaneously at the left common carotid and femoral arteries.24 PWV was the travel distance between the 2 transducers, measured on the body surface, divided by the transit time, determined manually by the foot-to-foot velocity method.24 For analysis, PWV measurements from 2 to 15 heart cycles were averaged. The directly measured path length (MONICA) was converted to the subtracted path length (IDCARS) in analyses involving both cohorts.7,25 For comparison of IDCARS and MONICA data in normal participants with a published meta-analysis,7 path length was converted to the path length considered to reflect the true anatomic distance, using published formula.7 The biochemical methods are available in the online-only Supplemental Material (p S5).
Ascertainment of End Point
The coprimary end points in the current study were a composite cardiovascular end point, including cardiovascular death and nonfatal cardiovascular events, and total mortality (TM). The secondary end points included cardiovascular mortality and fatal combined with nonfatal coronary events are defined in the Supplemental Material (pp S5–S6). In all outcome analyses, only the first event within each category was considered. Participants free of events were censored at last follow-up.
Statistical Analysis
Statistical methods are described in detail in the online-only Supplemental Material (pp S6–S9). In exploratory analyses, incidence rates of end points were tabulated by tertiles of the PWV distribution, while applying the direct method for standardizing rates in IDCARS for cohort, sex and age (<40, 40–59, ≥60 years), and for sex and age group (40, 50, 60, and 70 years) in MONICA. The cumulative incidence of the primary and secondary end points was plotted, while accounting for cohort (in IDCARS only) and sex and age (IDCARS) or age group (MONICA).
Multivariable-adjusted Cox models accounted for sex, age (IDCARS) or age group (MONICA), MAP, heart rate, body mass index, the total-to-high-density lipoprotein serum cholesterol ratio, smoking and drinking, use of antihypertensive drugs, history of diabetes,26 and previous cardiovascular disease (IDCARS only). Multivariable analyses involving IDCARS additionally accounted for cohort. The proportional hazards assumption was checked by the Kolmogorov-type supremum test. To compare relative risk across strata, deviation from mean coding27 was applied.
To determine an operational threshold for PWV, a 2-pronged strategy28,29 was applied using Cox regression in IDCARS. First, multivariable-adjusted hazard ratios (HRs) were computed for 0.1 m/s increments in PWV from the 10th to the 90th percentile of the PWV distribution. These HRs expressed the risk in participants, whose PWV exceeded the cutoff point versus the average risk in the whole population. The HRs with CIs were plotted as function of increasing PWV thresholds to assess at which PWV level the lower 95% CI of the HRs crossed unity, signifying increased risk.28 Next, PWV thresholds were obtained by determining the PWV levels yielding a 5-year risk equivalent to the risk associated with an office systolic BP of 120, 130, 140, and 160 mm Hg.29 Model calibration was evaluated by comparing the predicted risk against overoptimism-corrected Kaplan-Meier estimates in PWV quintiles. The performance of PWV in risk stratification was assessed from 2-by-2 tables providing specificity, sensitivity, and related statistics, the area under the curve, and by the integrated discrimination improvement (IDI) and the net reclassification improvement (NRI).30 Finally, subgroup analyses were conducted in participants stratified by sex, age (<60 versus ≥60 years), and the approximate median systolic BP in IDCARS and MONICA (<130 versus ≥130 mm Hg).
RESULTS
Baseline Characteristics
In IDCARS, the number of interpolated values amounted to 108 (3.2%) for total serum cholesterol, 198 (5.9%) for high-density lipoprotein cholesterol, 89 (2.6%) for blood glucose, 161 (4.8%) for smoking, and 723 (21.4%) for use of alcohol. In MONICA, the corresponding number of interpolated values were 1 (0.04%), 1 (0.04%), 5 (0.20%), 0 (0%), and 49 (1.99%), respectively.
The differences in the baseline characteristics between the IDCARS (2000–2016) and the MONICA (1993–1994) participants at the time of the vascular examinations (Table 1) reflect how lifestyle and treatment rates of hypertension changed over time, the ethnic make-up and the age structure of the discovery and replication cohorts, and the high tax rates levels levied on alcoholic beverages in Denmark. In both IDCARS and MONICA, women compared with men had smaller body height, lower body weight and body mass index, lower systolic and diastolic BP and MAP, and higher high-density lipoprotein cholesterol (Table S2). Across the IDCARS cohorts, mean PWV (SD) ranged from 7.2 (1.5) m/s in the Polish Gdańsk cohort to 8.9 (2.4) m/s in the participants recruited in Buenos Aires, Argentina (Figure S1). In the Copenhagen MONICA cohort, PWV averaged 8.0 (2.5) m/s (Figure S1).
Table 1.
Baseline Characteristics by Cohort
To assess concordance between the data resources used, normal individuals were sampled from IDCARS and MONICA by excluding patients with a history of cardiovascular disease, diabetes, or treated hypertension and by discarding patients with dyslipidemia and smokers (Figure S2). With standardization of the travel path applied, PWV by age group was largely similar in IDCARS and MONICA (Table S3). Furthermore, in single regression, the main correlates of PWV were age, systolic BP, pulse pressure, and MAP (Table S4) with Pearson correlation coefficients amounting to were 0.57, 0.45, 0.43, and 0.33 in IDCARS and to 0.55, 0.53, 0.48, and 0.47 in MONICA (P<0.001 for all).
Incidence of End Points
Median follow-up of IDCARS participants amounted to 4.3 years (interquartile range, 3.8–6.9 years; 5th to 95th percentile interval: 2.1–11.6 years). Over follow-up (Table S5), 105 participants (3.11%) died: 25 (0.74%) because of cardiovascular disease, 74 (2.19%) because of a non-cardiovascular illness (including kidney failure), and 6 (0.18%) because of nondocumented illnesses. The number of IDCARS participants experiencing a major cardiovascular event or a coronary end point amounted to 155 (4.59%) and 77 (2.28%), respectively.
Median follow-up in MONICA was 12.6 years (interquartile range, 12.2–13.1 years; 5th to 95th percentile interval: 3.5–13.4 years). Over this time period (Table S5), 393 (16.0%) died: 139 (5.66%) because of cardiovascular disease and 254 (10.3%) because of a non-cardiovascular ailment. The number of MONICA participants experiencing a major cardiovascular event or a coronary end point amounted to 354 (14.4%) and 202 (8.22%), respectively. Across tertiles of the PWV distribution, in IDCARS and in MONICA, the coprimary (Table S6) and secondary (Table S7) end points steeply increased with higher PWV category (P<0.001).
PWV Thresholds in IDCARS
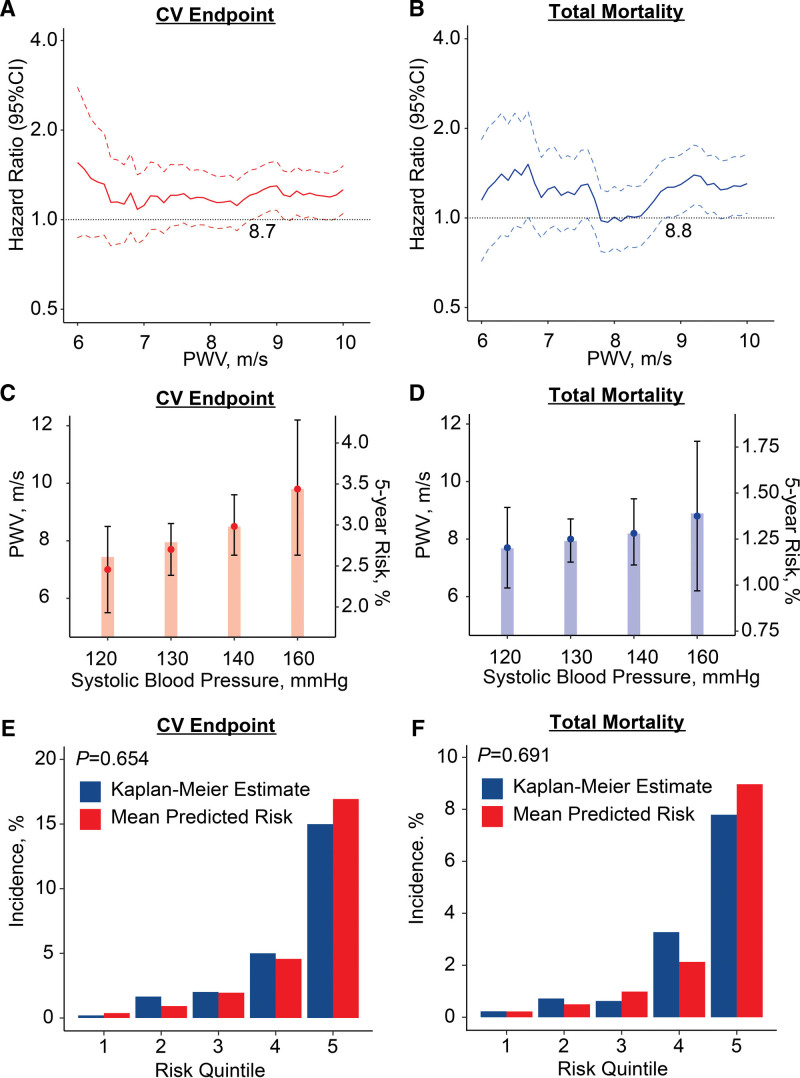
Multivariable-adjusted HRs were plotted against PWV thresholds stepwise increasing by 0.1 m/s over the 10th to 90th percentile range of the PWV distribution (Figure 1A and 1B). These multivariable-adjusted HRs expressed the 5-year risks of the coprimary end points associated with successively increasing PWV thresholds compared with the average risk in the whole IDCARS cohort. The lower limit of the 95% CI of the HRs crossed unity at a PWV level of 8.7 m/s for the composite cardiovascular end point and at 8.8 m/s for TM. In multivariable-adjusted Cox models (Figure 1C and 1D), the PWV thresholds yielding a risk equivalent with a systolic BP of 140 mm Hg were 8.5 m/s (CI, 7.5–9.6) for the composite cardiovascular end point and 8.2 m/s (7.1–9.4) for TM. In all models, PWV met the proportional hazard assumption (test statistic ≤1.26; P≥0.175). In IDCARS, with adjustments applied for cohort, sex, age, and MAP, a PWV threshold of <9 m/s versus ≥9 m/s separated (Figure S3) the cumulative incidence of the coprimary and secondary end points in a highly significantly way (P≤0.013). The fully adjusted HRs associated with a PWV ≥9 m/s versus <9 m/s were 1.68 (CI, 1.15–2.45) for the composite cardiovascular end point and 1.61 (1.01–2.55) for TM (Table 2); for cardiovascular mortality and coronary end points (Table S8), the corresponding HRs were 3.21 (1.06–9.69) and 2.08 (1.21–3.59), respectively. In Table 2 and Table S8, the HRs associated with a 1-SD PWV increment were also presented allowing comparison with the literature. The fully adjusted Cox models including the 9-m/s PWV threshold were well calibrated as evidenced by similarity between the Kaplan-Meier estimates and the multivariable-adjusted mean predicted risk for the cardiovascular end point (P=0.654) and TM (P=0.691) across quintiles of observed and predicted risk (Figure 1E and 1F). In subgroup analyses stratified for sex, age or median systolic BP, none of the interactions between the 9-m/s threshold and stratification groups reached significance (Figure S4).
Figure 1.
Threshold and calibration of pulse wave velocity (PWV) in 3378 IDCARS (International Database of Central Arterial Properties for Risk Stratification) participants. Hazard ratios (HRs) express the risk at each PWV level relative to the average risk in the whole study population for composite cardiovascular (CV) end point (A) and total mortality (B) with PWV at 8.7 and 8.8 m/s signifying increased risk by crossing unity (dotted line). PWV levels yielding equivalent 5-year risks compared with systolic blood pressure categories for composite cardiovascular end point (C) and total mortality (D) with bars indicating 5-year risks and point and line for PWV thresholds. PWV levels at 8.5 and 8.2 m/s indicate equivalent risk as a systolic blood pressure of 140 mm Hg. Model calibration for the composite cardiovascular end point (E) and total mortality (F), showing the predicted risk against overoptimism-corrected Kaplan-Meier estimates in PWV quintiles. All analyses were multivariable adjusted for cohort, sex, age, mean arterial pressure (excluding C and D), heart rate, the total-to-high-density lipoprotein serum cholesterol ratio, smoking and drinking, use of antihypertensive drugs, diabetes, and history of cardiovascular disease.
Table 2.
Coprimary End Points in Relation to PWV per Threshold and Analyzed as Continuously Distributed Variable
Replication in MONICA
The MONICA data were interrogated to replicate the clinical relevance of the proposed 9-m/s threshold. In line with the IDCARS findings, with adjustments applied for sex, age group, and MAP, a PWV threshold of <9 m/s versus ≥9 m/s differentiated (Figure S5) the cumulative incidence of the co-primary and secondary end points in a significant manner (P≤0.001), except for coronary event (P=0.145). The fully adjusted HRs associated with a PWV ≥9 m/s versus <9 m/s, were 1.40 (1.09–1.79) for the cardiovascular end point and 1.55 (1.23–1.95) for TM (Table 2); for cardiovascular mortality (Table S8) the HR was 1.53 (1.04–2.25), and for coronary events it was not significant (P=0.553). In subgroup analyses stratified for sex, age, or median systolic BP, none of the interactions between the 9-m/s threshold and the stratification groups reached significance (Figure S4).
Predictive Performance
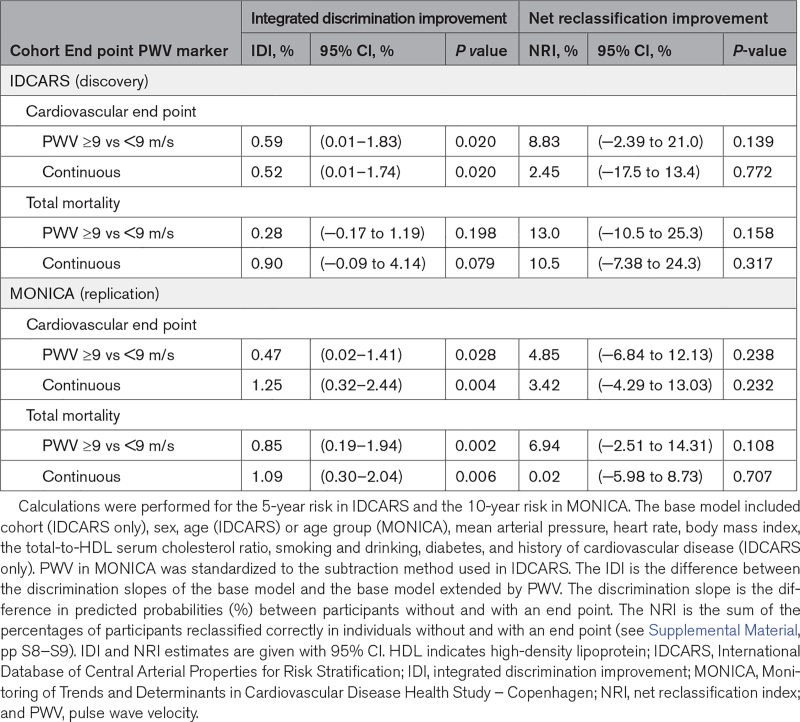
In IDCARS, specificity, sensitivity, and overall accuracy of the categorized PWV for the prediction of the cardiovascular end point were 0.775, 0.607, and 0.769 and for the prediction of death 0.767, 0.571, and 0.764, respectively. Estimates in MONICA were of similar magnitude (Table 3). In IDCARS, IDI for the 9-m/s PWV threshold amounted to 0.59% for the cardiovascular end point (P=0.020) and to 0.28% (P=0.198) for TM, while in MONICA the corresponding IDI values were 0.47 (P=0.028) and 0.85 (P=0.002), respectively (Table 4). However, none of the NRI estimates in IDCARS or MONICA reached statistical significance (P≥0.108).
Table 3.
Discriminative Performance of Pulse Wave Velocity
Table 4.
Integrated Discrimination Improvement and Net Reclassification Improvement by Adding Pulse Wave Velocity per Threshold and as Continuously Distributed Variable to the Base Model
Rescaling of the PWV Threshold
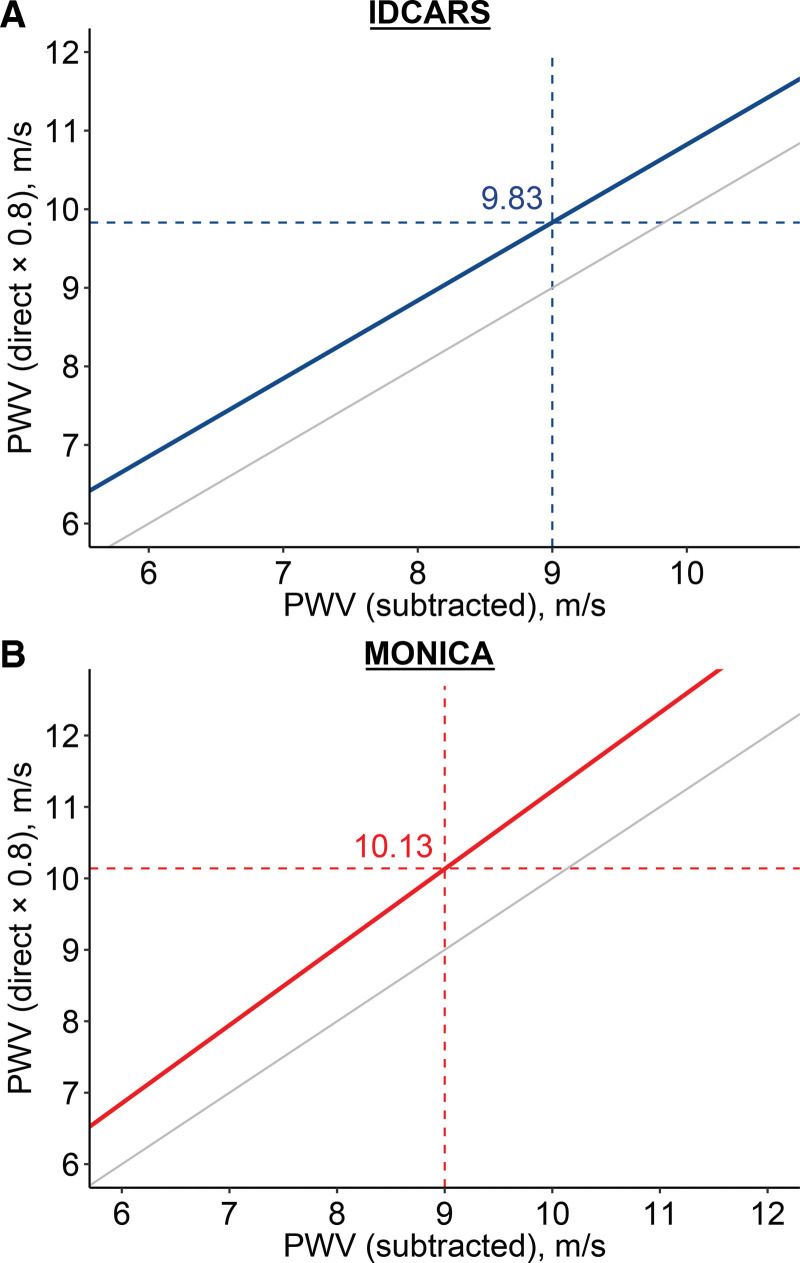
In the analysis of the IDCARS and Copenhagen MONICA data, the pulse wave travel distance was standardized to the subtraction method, as applied in IDCARS. To keep consistency with the current guidelines and the software presently implemented in devices for PWV measurement, the 9-m/s threshold derived in IDCARS and replicated in MONICA was rescaled to account for the difference between the measured and anatomic pulse wave travel path. With this adjustment applied the 9-m/s threshold corresponded with 10 m/s (Figure 2).
Figure 2.
Rescaling the outcome-driven pulse wave threshold for the anatomical pulse wave travel distance path. In the analysis of the IDCARS (International Database of Central Arterial Properties for Risk Stratification) and the Copenhagen MONICA (Monitoring of Trends and Determinants in Cardiovascular Disease Health Survey – Copenhagen) data, the pulse wave travel distance was standardized to the subtraction method, as applied in IDCARS. To keep consistency with the current guidelines and clinical practice, the 9-m/s threshold derived in IDCARS and replicated in MONICA was rescaled to account for the difference between the measured and anatomic pulse wave travel path, using the formula published in references.2,7 With this adjustment applied the 9-m/s threshold corresponded with 10 m/s. The gray line represents the line of identity. PWV indicates pulse wave velocity.
DISCUSSION
In IDCARS, a 2-pronged approach was applied to determine outcome-driven PWV thresholds in relation to the cardiovascular end point and TM. The PWV thresholds in well-calibrated models converged to 9 m/s, of which the prognostic value was replicated in MONICA. The 2007 European Guideline for the Management of Hypertension3 proposed a risk-carrying PWV threshold of 12 m/s, because this level was believed to represent a rough estimate of high cardiovascular risk. The 2012 Consensus Document on the Measurement of PWV2 referred to the longitudinal patient and population studies published at that time to justify the 12-m/s threshold. However, the document2 went on stating that the 12-m/s cut-off limit was based on the direct measurement of the pulse transit distance. It therefore proposed a new standard distance, that is, (common carotid artery−common femoral artery)×0.8.6 Applying the new standard6 would result in a PWV threshold of 9.6 m/s, which was rounded to 10 m/s as an easy to remember value for use in daily clinical practice.2 In the current study, where relevant, the directly measured travel distance (MONICA) was converted to the subtraction distance (IDCARS)2,7 to increase comparability of the PWV estimates, either as descriptive variable (Figure S1) or as exposure variable. Accounting for the anatomic pulse wave travel distance showed that the 9-m/s threshold was equivalent with the 10-m/s cutoff, as proposed in the European guidelines.3,7
Several results presented in the current article were generated as validation of the data resources being used. First, the sex distribution of anthropometric and hemodynamic characteristics and serum lipids was in line with the literature. In both IDCARS and MONICA, women compared with men had smaller body height, lower body weight and body mass index, lower systolic and diastolic BP and MAP, and higher high-density lipoprotein cholesterol (Table S2). Second, the main correlates of PWV were age, systolic BP, pulse pressure, and MAP (Table S4). Third, to assess concordance with the literature, normal individuals were sampled from the IDCARS and the MONICA cohorts, using the same exclusion criteria as described by The Reference Values for Arterial Stiffness’ Collaboration (Figure S2).7 With standardization of the pulse wave travel path applied,2,7 PWV by age group was largely similar in IDCARS and MONICA and in agreement with the normal subgroup in the previously published cross-sectional meta-analysis (Table S3).
Clinical Significance
Modelling time-to-event using proportional hazard regression implies that the association between adverse health outcomes and a risk factor is log-linear without a threshold at which the risk suddenly increases. Population studies of office31 or out-of-office32 BP or serum cholesterol33 have unmistakably illustrated this concept. Given that in the present analysis, the proportional hazard assumption for PWV was met, and this construct is also applicable to PWV. For this reason, throughout the current article, the risk with PWV was not only tabulated for the 9-m/s threshold, but also for a 1-SD increment in the continuously distributed PWV. Notwithstanding the continuous associations between adverse health outcomes and risk factors, operational or actionable thresholds of risk factors support clinicians in risk stratification and in identifying the need to start pharmacological treatment. Both in IDCARS and MONICA (Table 3), specificity of the 9-m/s threshold for the coprimary end points was ≈0.80, sensitivity was ≈0.55, and the overall predictive accuracy close to 0.75. IDI was significant for the cardiovascular end point in IDCARS and both coprimary end points in MONICA, whereas NRI did not reach statistical significance. The multivariable-adjusted IDI and NRI provide complementary information. Indeed, if addition of a marker to a model including several risk factors increases the predicted probability of an end point, this is reflected by a significant increase in IDI (Table 4). NRI indicates the extent to which a biomarker improves diagnostic accuracy, which in the current analyses was not significant, indicating that the discriminatory performance of PWV on top of commonly measured risk factors, in particular sex, age, various BP indexes (Table S4), and dyslipidemia, is small. A risk calculator is made available as Supplemental Material 2. The SPARTE (Strategy for Preventing Cardiovascular and Renal Events Based on Arterial Stiffness) Investigators34 and the pathophysiology of aortic stiffness35–37 provide the interpretation of these findings. Aortic stiffness, as captured by PWV, integrates the lifetime injury to the arterial wall. Elastin and collagen are the major constituents of the extracellular matrix in the media of the central elastic arteries. Elastin provides reversible extensibility during systole, while collagen generates tensile strength. As people age, the elastin fibers become fragmented and the mechanical load is transferred to collagen fibers, which are up to 1000 times stiffer than elastin.35 This process already starts in young adulthood, but the deposition of elastin by vascular smooth muscle cells only occurs during fetal development and in early infancy, and is switched-off thereafter.36 This implies that elastin fiber damage is basically irreversible.37
In the SPARTE trial,34 hypertensive patients were randomized to a therapeutic strategy targeting the normalization of PWV, measured every 6 months (N=264) or to a therapeutic strategy only implementing the European Hypertension Guidelines3 (N=272). After a median follow-up of 48.3 months, there was no significant between-group difference in the primary outcome, a composite cardiovascular end point (HR, 0.74 [CI, 0.40–1.38]). However, the secondary end points were met by showing that PWV-driven treatment for hypertension reduces office and ambulatory BP and aortic stiffening more than with application of BP-based guidelines. In a subgroup of 337 participants enrolled in SPRINT (Systolic Blood Pressure Intervention Trial; 45% women; mean age, 64 years),38 intensive treatment (target systolic BP <120 mm Hg) compared with usual treatment (<140 mm Hg) produced a mean between-group reduction in systolic BP of 12.7 mm Hg (CI, 11.1–14.3 mm Hg) and at the end of the 18-month follow-up had attenuated the increase in PWV (9.0 versus 10 m/s; P<0.001). Both the trials highlighted the pathophysiological concept that age and high BP are the main drivers of aortic stiffening. However, clinicians should be particularly concerned about patients, in whom there is disparity between PWV, age, and MAP, and retrace the previous and current medical history of such patients to identify hidden risk factors. In the context of the current study, a PWV risk threshold of 9 m/s (or 10 m/s with the correction for the anatomic pulse wave travel path applied) should motivate clinicians to achieve stringent control of BP, in particular systolic BP, the extending force to be buffered by the elastin fibers.
Limitations
The current study should be carefully interpreted within the context of its limitations. First, the diagnostic criteria and invasive management of coronary heart disease improved drastically from the early 1990s to the current state of the art. In MONICA, only a single case of coronary revascularization was registered, whereas this number in IDCARS was 57 (Table S5). These period effects might explain why the 9-m/s PWV threshold was not replicated for coronary end points in MONICA, whereas PWV analyzed as continuously distributed variable retained significance (Table S8). Second, as shown by the NRI, the incremental value associated with PWV on top of all other risk factors was not significant (Table 4), explaining why the NRI results were not graphically translated into an analysis of the area under the curve of nested models. Finally, although IDCARS was a multiethnic cohort, Black people were not represented in the current data resource.
Perspectives
This individual-participants meta-analysis of longitudinal population studies with a composite cardiovascular end point and TM as coprimary end points identified, validated, and replicated 9 m/s as new outcome-driven threshold for aortic PWV. With correction for the anatomic travel path, this cut-off corresponds with the 10-m/s threshold proposed in European guidelines.3,7 In quantitative terms, these outcome-driven thresholds refine risk stratification (IDI), albeit with a nonsignificant amount (NRI). In settings where PWV measurement can be implemented, exceeding the actionable thresholds should motivate clinicians to stringent management of modifiable cardiovascular risk factors, in particular systolic BP, which over a person’s lifetime leads to elastin fragmentation in the wall of the central arteries, thereby causing major cardiovascular complication and premature mortality.
ARTICLE INFORMATION
Acknowledgments
Additional Information: This article is dedicated to the memory of Gavin R. Norton, MD, PhD, who passed away on December 9, 2022 shortly after having attended the IDCARS Consortium Meeting (Mechelen, Belgium, December 1–3, 2022) and whose comprehensive understanding of arterial physiology inspired all authors of this article. IDCARS investigators: Argentina, Buenos Aires: LS Aparicio, J Barochiner; Belgium, Noordkempen: D-M Wei, JD Melgarejo, L Thijs, JA Staessen, F-F Wei, W-Y Yang, Z-Y Zhang; China, Jing Ning: DW An, YB Cheng, QH Guo, JF Huang, QF Huang, Y Li, CS Sheng, JG Wang; Czech Republic, Pilsen: J Filipovský, J Seidlerová; Finland, FinRisk: EP Juhanoja, AM Jula, AS Lindroos, TJ Niiranen, SS Sivén; Italy, Padova: E Casiglia, A Pizzioli, V Tikhonoff; Nigeria, Abuja: BS Chori, B Danladi, AN Odili, H Oshaju; Poland, Gdańsk: W Kucharska, K Kunicka, N Gilis-Malinowska, K Narkiewicz, W Sakiewicz, E Swierblewska; Poland, Kraków: K Kawecka-Jaszcz, K Stolarz-Skrzypek, M Rajzer; South Africa, Potchefstroom: C Mels, R Kruger, G Mokwatsi, AE Schutte; South Africa, Johannesburg: GR Norton, AJ Woodiwiss; Switzerland, Bern, Geneva and Lausanne: D Ackermann, M Bochud, G Ehret; Uruguay, Montevideo: R Álvarez-Vaz, C Américo, C Baccino, L Borgarello, L Florio, P Moliterno, A Noboa, O Noboa, A Olascoaga, P Parnizari, M Pécora.
Sources of Funding
The Non-Profit Research Association Alliance for the Promotion of Preventive Medicine, Mechelen, Belgium (www.appremed.org) received a nonbinding grant from OMRON Healthcare Co. Ltd., Kyoto, Japan, which supports the scholarships of D.-W. An, B. Chori, and Y.-L. Yu. The grants that supported the cohort studies are listed by country. Argentina: The Internal Medicine Service, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina. Belgium: European Union (HEALTH-F7-305507 HOMAGE), European Research Council (Advanced Researcher Grant 2011-294713-EPLORE and Proof-of-Concept Grant 713601-uPROPHET), European Research Area Net for Cardiovascular Diseases (JTC2017-046-PROACT), and Research Foundation Flanders, Ministry of the Flemish Community, Brussels, Belgium (G.0881.13). China: The National Natural Science Foundation of China (grants 82270469, 82070432, and 82070435), the Ministry of Science and Technology (2018YFC1704902), Beijing, China, and by Shanghai Municipal Health Commission (2022LJ022 and 2017BR025). Czech Republic: European Union (grants LSHM-CT−2006 to 037093 and HEALTH-F4−2007 to 201550) and Charles University Research program, “Cooperatio – Cardiovascular Science.” Denmark: 01-2-9-9A-22914 from the Danish Heart Foundation and R32-A2740 from the Lundbeck Fonden. Finland: Academy of Finland (grant 321351), Emil Aaltonen Foundation, the Paavo Nurmi Foundation, the Urmas Pekkala Foundation, and the Hospital District of South-Western Finland. Italy: European Union (grants LSHM-CT−2006 to 037093 and HEALTH-F4−2007 to 201550). Poland (Gdańsk): European Union (grants LSHM-CT−2006 to 037093 and HEALTH-F4−2007 to 201550). Poland (Kraków): European Union (grants LSHM-CT−2006 to 037093 and HEALTH-F4−2007 to 201550) and Foundation for Polish Science.
Disclosures
None.
Supplemental Material
Expanded Methods
Table S1–S8
Figures S1–S5
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BP
- blood pressure
- HR
- hazard ratio
- IDCARS
- International Database of Central Arterial Properties for Risk Stratification
- IDI
- integrated discrimination improvement
- MAP
- mean arterial pressure
- MONICA
- Monitoring of Trends and Determinants in Cardiovascular Disease Health Survey – Copenhagen
- NRI
- net reclassification improvement
- PWV
- pulse wave velocity
- TM
- total mortality
D.-W. An and T. Hansen are joint first authors and contributed equally.
J. Staessen and Y. Li are joint senior co-corresponding authors and contributed equally.
The International Database of Central Arterial Properties for Risk Stratification Investigators are listed in the Acknowledgments.
For Sources of Funding and Disclosures, see page 1958.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.123.21318.
REFERENCES
- 1.Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:1237–1263. doi: 10.1016/j.jacc.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, et al. ; Artery Society. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–448. doi: 10.1097/HJH.0b013e32834fa8b0 [DOI] [PubMed] [Google Scholar]
- 3.Mancia G, De BG, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, et al. 2007 ESH-ESC practice Guidelines for the management of arterial hypertension: ESH-ESC task force on the management of arterial hypertension. J Hypertens. 2007;25:1751–1762. doi: 10.1097/HJH.0b013e3282f0580f [DOI] [PubMed] [Google Scholar]
- 4.Joint Committee for Guideline Revision. 2018 Chinese guidelines for prevention and treatment of hypertension — a report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J Geriatr Cardiol. 2022;16:182–241. doi: 10.11909/j.issn.1671-5411.2019.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. ; ESC Scientific Document Group. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 6.Huybrechts SAM, Devos DG, Vermeersch SJ, Mahieu D, Achten E, de Backer TLM, Segers P, Van Bortel LM. Carotid to femoral pulse wave velocity: a comparison of real travelled aortic path lengths determined by MRI and superficial measurements. J Hypertens. 2011;29:1577–1582. doi: 10.1097/HJH.0b013e3283487841 [DOI] [PubMed] [Google Scholar]
- 7.The Reference Values for Arterial Stiffness’ collaboration. Determinants of pulse wave velocity in healthy people and the presence of cardiovascular risk factors: “establishing normal and reference values”. Eur Heart J. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117. doi: 10.1161/01.hyp.33.5.1111 [DOI] [PubMed] [Google Scholar]
- 9.London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434 [DOI] [PubMed] [Google Scholar]
- 10.Pannier B, Guérin AP, Marchais SJ, Safar ME, London GM. Stiffness of capacitive and conduit arteries. Prognostic significance for end-stage renal disease patients. Hypertension. 2005;45:592–596. doi: 10.1161/01.HYP.0000159190.71253.c3 [DOI] [PubMed] [Google Scholar]
- 11.Adragâo T, Pires A, Birne R, Dias Curto J, Lucas C, Gonçalves M, Pita Negrâo A. A plain X-ray vascular calcification score is associated with arterial stiffness and mortality in dialysis patients. Nephrol Dial Transplant. 2009;24:997–1002. doi: 10.1093/ndt/gfn584 [DOI] [PubMed] [Google Scholar]
- 12.Avramovski P, Janakievska P, Sotiroski K, Zafirova-Ivanovska B, Sikole A. Aortic pulse wave velocity is a strong predictor of all-cause and cardiovascular mortality in chronic dialysis patients. Ren Fail. 2013;36:176–186. doi: 10.3109/0886022x.2013.843359 [DOI] [PubMed] [Google Scholar]
- 13.Broyd CJ, Patel K, Pugliese F, Chebab O, Mathur A, Baumbach A, Ozkor M, Kennon S, Mullen M. Pulse wave velocity can be accurately measured during transcatheter aortic calve implantation and used for post-procedure risk stratification. J Hypertens. 2019;37:1845–1852. doi: 10.1097/HJH.0000000000002141 [DOI] [PubMed] [Google Scholar]
- 14.Ryliškyte L, Navickas R, Šerpytis P, Puronaite R, Zupkauskiene J, Juceviciene A, Badariene J, Rimkiene MA, Ryliškiene K, Skiauteryte E, et al. Association of aortic stiffness, carotid intima-media thickness and endothelial function with cardiovascular events in metabolic syndrome subjects. Blood Press. 2019;28:131–138. doi: 10.1080/08037051.2019.1569461 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q, Yin K, Zhu M, Lin X, Fang Y, Lu J, Li Z, Ni Z. Combining pulse wave velocity with galectin-3 to predict mortality and cerebrovascular and cardiovascular events in hemodialysis patients. Front Med. 2020;7:579021. doi: 10.3389/fmed.2020.579021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shokawa T, Imazu M, Yamamoto H, Toyofuku M, Tasaki N, Okimoto T, Yamane K, Kohno N. Pulse wave velocity predicts cardiovascular mortality: findings from the Hawaii-Los Angeles-Hiroshima study. Circ J. 2005;69:259–264. doi: 10.1253/circj.69.259 [DOI] [PubMed] [Google Scholar]
- 17.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, et al. ; Health ABC Study. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628 [DOI] [PubMed] [Google Scholar]
- 18.Inoue N, Maeda R, Kawakami H, Shokawa T, Yamamoto H, Ito C, Sasaki H. Aortic pulse wave velocity predicts cardiovascular mortality in middle-aged and elderly Japanse men. Circ J. 2009;73:549–553. doi: 10.1253/circj.cj-08-0492 [DOI] [PubMed] [Google Scholar]
- 19.Sehestedt T, Jeppesen J, Hansen TW, Rasmussen S, Wachtell K, Ibsen H, Torp Pedersen C, Olsen MH. Thresholds for pulse wave velocity, urinary albumin creatinine ratio and left ventricular mass index using SCORE, Framingham and ESH/ESC risk charts. J Hypertens. 2012;30:1928–1936. doi: 10.1097/HJH.0b013e328356c579 [DOI] [PubMed] [Google Scholar]
- 20.Takami T, Shigemasa M. Efficacy of various antihypertensive agents as evaluated by indices of vascular stiffness in elderly hypertensive patients. Hypertens Res. 2003;26:609–614. doi: 10.1291/hypres.26.609 [DOI] [PubMed] [Google Scholar]
- 21.Aparicio LS, Huang QF, Melgarejo JD, Wei DM, Thijs L, We FF, Gilis-Malinowska N, Sheng CS, Boggia J, Niiranen TJ, et al. The International Database of Central Arterial Properties for Risk Stratification: research objectives and baseline characteristics of participants. Am J Hypertens. 2022;35:54–64. doi: 10.1093/ajh/hpab139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen TW, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342 [DOI] [PubMed] [Google Scholar]
- 23.World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. J Am Med Assoc. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 24.Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R, Levy BI. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485–490. doi: 10.1161/01.hyp.26.3.485 [DOI] [PubMed] [Google Scholar]
- 25.Vermeersch SJ, Rietzschel ER, De Buyere ML, Van Bortel LM, Gillebert TC, Verdonck PR, Laurent S, Segers P, Boutouyrie P. Distance measurements for the assessment of carotid to femoral pulse wave velocity. J Hypertens. 2009;27:2377–2385. doi: 10.1097/HJH.0b013e3283313a8a [DOI] [PubMed] [Google Scholar]
- 26.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabet Care. 2003;26(Suppl. 1):S5–S20. doi: 10.2337/diacare.26.2007.s5 [DOI] [PubMed] [Google Scholar]
- 27.Hosmer DW, Jr, Leleshow S. Applied Logistic Regression. New York, NY: John Wiley & Sons; 1989. [Google Scholar]
- 28.Gu YM, Thijs L, Li Y, Asayama K, Boggia J, Hansen TW, Liu YP, Ohkubo T, Bjorklund-Bodegard K, Jeppesen J, et al. ; International Database on Ambulatory blood pressure in relation to Cardiovascular Outcomes (IDACO) Investigators. Outcome-driven thresholds for ambulatory pulse pressure in 9938 participants recruited from 11 populations. Hypertension. 2014;63:229–237. doi: 10.1161/HYPERTENSIONAHA.113.02179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kikuya M, Hansen TW, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, Richart T, Torp-Pedersen C, Lind L, Ibsen H, et al. ; International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes Investigators. Diagnostic thresholds for ambulatory blood pressure monitoring based on 10-year cardiovascular risk. Circulation. 2007;115:2145–2152. doi: 10.1161/CIRCULATIONAHA.106.662254 [DOI] [PubMed] [Google Scholar]
- 30.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72; discussion 207. doi: 10.1002/sim.2929 [DOI] [PubMed] [Google Scholar]
- 31.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Thijs L, Zhang ZY, Asayama K, Hansen TW, Boggia J, Björklund-Bodegård K, Yang WY, Niiranen TJ, Ntineri A, et al. ; International Database on Ambulatory and Home Blood Pressure in Relation to Cardiovascular Outcome Investigators. Opposing age-related trends in absolute and relative risk of adverse health outcomes associated with out-of-office blood pressure. Hypertension. 2019;74:1333–1342. doi: 10.1161/HYPERTENSIONAHA.119.12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asia Pacific Cohort Studies Collaboration. Cholesterol, coronary heart disease, and stroke in the Asia Pacific region. Intern J Epidemiol. 2003;N32:563–572. doi: 10.1093/ije/dyg106 [DOI] [PubMed] [Google Scholar]
- 34.Laurent S, Chatellier G, Azizi M, Calvet D, Choukroun G, Danchin N, Delsart P, Girerd X, Gosse P, Khettab H, et al. ; SPARTE Investigators. SPARTE Study: normalization of arterial stiffness and cardiovascular events in patients with hypertension at medium to very high risk. Hypertension. 2021;78:983–995. doi: 10.1161/HYPERTENSIONAHA.121.17579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O’Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68:50–58. doi: 10.1161/01.cir.68.1.50 [DOI] [PubMed] [Google Scholar]
- 36.Wagenseil J, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–989. doi: 10.1152/physrev.00041.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagenseil J, Mecham RP. Elastin in large artery stiffness and hypertension. J Cardiovasc Trans Res. 2012;5:264–273. doi: 10.1007/s12265-012-9349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Upadhya B, Pajewski NM, Rocco MV, Hundley WG, Aurigemma G, Hamilton CA, Bates JT, He J, Chen J, Chonchol M, et al. ; SPRINT Research Group. Effect of intensive blood pressure control on aortic stiffness in the SPRINT-HEART. Hypertension. 2021;77:1571–1580. doi: 10.1161/HYPERTENSIONAHA.120.16676 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All available data are shown within the article and the online-only Supplemental Material. Anonymized data are available from the corresponding author upon request; on condition that an analysis plan is accompanying the request and that the principal investigators of all cohorts approve data sharing.