Abstract
In this multicenter, single-arm phase 2 trial (ChiCTR1900024428), patients with locally advanced gastric/gastroesophageal junction cancers receive one cycle of sintilimab (anti-PD1) and chemotherapy (S-1 and nab-paclitaxel), followed by 5 weeks of concurrent chemoradiotherapy and sintilimab, and another cycle of sintilimab and chemotherapy thereafter. Surgery is preferably scheduled within one to three weeks, and three cycles of adjuvant sintilimab and chemotherapy are administrated. The primary endpoint is the pathological complete response. Our results meet the pre-specified primary endpoint. Thirteen of 34 (38.2%) enrolled patients achieve pathological complete response (95% CI: 22.2-56.4). The secondary objectives include disease-free survival (DFS), major pathological response, R0 resection rate, overall survival (OS), event-free survival (EFS), and safety profile. The median DFS and EFS were 17.0 (95%CI: 11.1-20.9) and 21.1 (95%CI: 14.7-26.1) months, respectively, while the median OS was not reached, and the 1-year OS rate was 92.6% (95%CI: 50.1-99.5%). Seventeen patients (50.0%) have grade ≥3 adverse events during preoperative therapy. In prespecified exploratory biomarker analysis, CD3+ T cells, CD56+ NK cells, and the M1/M1 + M2-like macrophage infiltration at baseline are associated with pathological complete response. Here, we show the promising efficacy and manageable safety profile of sintilimab in combination with concurrent chemoradiotherapy for the perioperative treatment of locally advanced gastric/gastroesophageal junction adenocarcinoma.
Subject terms: Cancer therapy, Gastroenterology, Gastric cancer, Tumour immunology
Neoadjuvant chemotherapy followed by gastrectomy is considered standard of care for locally advanced gastric and gastroesophageal junction (G/GEJ) cancers. Here the authors report the results of a phase 2 trial of neoadjuvant sintilimab (anti-PD1) plus chemoradiotherapy in patients with locally advanced G/GEJ tumors.
Introduction
Gastric and gastroesophageal junction (G/GEJ) cancers represent the fifth most common newly diagnosed malignancy and the fourth leading cause of cancer-related death worldwide1. In China, approximately 400,000 patients were diagnosed with G/GEJ cancers, and 289,000 individuals died of G/GEJ cancers in 20162. Furthermore, nearly half of the gastric cancers in China are stage III or IV at diagnosis3. Despite recent advances in multidisciplinary or multimodal therapies, the prognosis of locally advanced patients remains poor, with a median overall survival (OS) of only 34.4 months and a 5-year OS rate of only 38.7%4.
According to the guidelines of the Chinese Society of Clinical Oncology (CSCO), a combination of perioperative therapy and D2 gastrectomy is currently considered the standard treatment option for locally advanced G/GEJ cancers (cT3N2-3M0 and cT4aN+M0, or cT4bNanyM0 after multidisciplinary discussion)5. Neoadjuvant chemotherapy based on fluorouracil, platinum, taxanes, or chemoradiotherapy followed by surgery and adjuvant chemotherapy is recommended by National Comprehensive Cancer Network (NCCN) and the CSCO guidelines5,6. Nevertheless, the optimal perioperative therapeutic protocol and sequence remain undefined. Although perioperative therapy can lead to a high R0 resection rate of more than 90%7–9. Therefore, the optimization of perioperative treatment regimens is warranted to improve the clinical outcomes of patients with locally advanced G/GEJ cancers.
Immune checkpoint inhibitors that target programmed cell death protein 1 (PD-1) or programmed cell death ligand 1 (PD-L1) have shown promising survival benefits and manageable safety in first-line treatment of patients with G/GEJ cancers in the CheckMate-64910, GEMSTONE-10111, and ORIENT-16 trials12. By binding to PD-1, activating antigen-specific T cells, and reversing the immune evasion of cancer, immunotherapy may be more effective in the early stages of G/GEJ cancers when the tumor is still present after the approach known as neoadjuvant therapy13. Although the survival benefit of neoadjuvant immunotherapy has been demonstrated in several solid tumors, including breast cancer14 and hepatocellular carcinoma15, lung cancer16, and melanoma17, there is limited evidence in G/GEJ cancers.
In recent studies, it has been demonstrated that conventional cancer therapies, including chemotherapy and radiotherapy, alter the tumor immune microenvironment (TiME) (e.g., increasing the expression levels of immune checkpoints18), which is crucial for the development, progression, and therapeutic responses of tumors19. Besides, preclinical studies indicated the synergistic effect of concurrent chemoradiotherapy (cCRT) on immunotherapy by enhancing the host immune response and inhibiting cancer cell immune escape20,21. The CheckMate-577 study demonstrated the survival benefit of adjuvant nivolumab in patients with esophageal/GEJ cancer who received preoperative chemoradiotherapy22. However, the efficacy and safety of perioperative immunotherapy plus cCRT in locally advanced gastric cancer remain mostly unexplored23.
Sintilimab, a fully human and highly selective anti-programmed death (PD)-1 monoclonal antibody, binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, causing a robust antitumor effect24. In the ORIENT-16 study, first-line sintilimab plus chemotherapy improved survival in Chinese patients with advanced G/GEJ cancers12. There is evidence of the efficacy of paclitaxel in gastric cancer25 and of nab-paclitaxel in the second- and third-line treatment of gastric cancer26–28. There is also evidence of the non-inferiority efficacy of nab-paclitaxel vs. paclitaxel in previously treated advanced gastric cancer29 and significantly longer progression-free survival of additional nab-paclitaxel vs. paclitaxel to S-1 in the first line treatment of advanced gastric cancer30. In addition, nab-paclitaxel does not require premedication with corticosteroids. In Japan, D2 gastrectomy and adjuvant S-1 are the standard management of locally advanced gastric cancer31. The combination of nab-paclitaxel and S-1 has been demonstrated as an effective and safe first-line treatment28.
Hence, in this multicenter, single-arm phase 2 trial, we aim to evaluate the efficacy and safety of perioperative sintilimab plus cCRT in patients with locally advanced G/GEJ cancers. Further, we conduct an exploratory multiplex immunofluorescence (mIF) analysis of TiME with the aim of identifying promising biomarkers of treatment response. Here, we show the promising efficacy and manageable safety profile of sintilimab in combination with concurrent chemoradiotherapy for the perioperative treatment of locally advanced G/GEJ adenocarcinoma.
Results
Patient characteristics
From July 20, 2019 to October 10, 2021, 42 patients were screened for eligibility, and 8 were excluded due to refusal to participate (n = 4), peritoneal metastasis (n = 3), and a history of severe rash (n = 1, Supplementary Fig. 1). Finally, a total of 34 patients were enrolled, with a median age of 65.5 years (range, 58–68). Among them, 28 patients (82.4%) were male, and 26 (76.5%) had an ECOG PS score of 0. Thirty-one patients (91.2%) were diagnosed with gastric cancer, and 3 (8.8%) had GEJ cancer. Three (8.8%), 18 (52.9%), 7 (20.6%), and 6 (17.7%) cases were Bormann type I, II, III, and IV, respectively. Detailed patient baseline characteristics are presented in Table 1.
Table 1.
Baseline patient characteristics
| Characteristic | Total (n = 34) |
|---|---|
| Age, years, median (range) | 65.5 (58–68) |
| Male, n (%) | 28 (82.4) |
| ECOG PS score of 0, n (%) | 26 (76.5) |
| Diagnosis, n (%) | |
| Gastric cancer | 31 (91.2) |
| GEJ cancer | 3 (8.8) |
| Bormann subtype, n (%) | |
| I | 3 (8.8) |
| II | 18 (52.9) |
| III | 7 (20.6) |
| IV | 6 (17.7) |
| Clinical T categorya, n (%) | |
| T3 | 10 (29.4) |
| T4a | 19 (55.9) |
| T4b | 5 (14.7) |
| Clinical N categorya, n (%) | |
| N1 | 1 (2.9) |
| N2 | 21 (61.8) |
| N3 | 12 (35.3) |
| Histologic grade, n (%) | |
| G2 | 12 (35.3) |
| G3 | 22 (64.7) |
| Signet ring cell carcinoma, n (%) | 4 (11.8) |
| Lauren’s classification, n (%) | |
| Intestinal type | 17 (50.0) |
| Diffuse type | 10 (29.4) |
| Mixed type | 6 (17.7) |
| Unknown | 1 (2.9) |
| PD-L1 CPS, n (%) | |
| <1 | 16 (47.1) |
| ≥1 and <5 | 5 (14.7) |
| ≥5 | 11 (32.4) |
| Unknown | 2 (5.9) |
| MSI/MMR status, n (%) | |
| MSI-H/dMMR | 1 (2.9) |
| MSS/pMMR | 33 (97.1) |
| HER2 status, n (%) | |
| 0 | 19 (55.9) |
| 1+ | 10 (29.4) |
| 2+ | 5 (14.7) |
ECOG PS Eastern Cooperative Oncology Group Performance Status, GEJ gastroesophageal junction, MSS microsatellite stable, pMMR proficient mismatch repair, CPS combined proportional score, PD-L1 programmed cell death ligand 1.
aPathological tumor and lymph-node statuses were classified according to the criteria proposed by the eighth edition of the Cancer Staging Manual of the American Joint Committee on Cancer.
Pathological responses
All patients received neoadjuvant therapy and underwent surgery. Thirty-three (97.1%) patients underwent total gastrectomy plus D2 lymphadenectomy, and 1 (2.9%) underwent proximal gastrectomy with D2 lymphadenectomy. The rate of R0 resection was 100%. Thirty-one (91.2%) patients underwent adjuvant therapy, while 3 did not due to refusal (n = 2) and hepatic toxicity (n = 1).
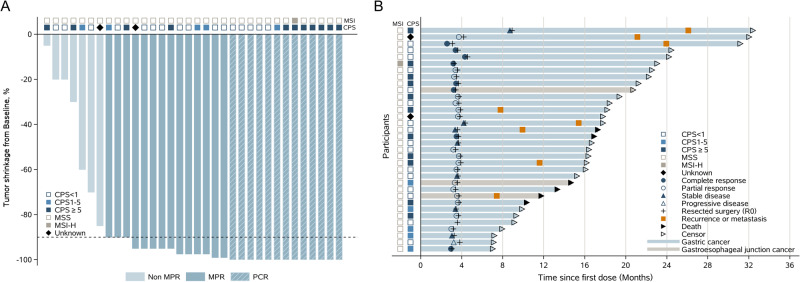
Tumor shrinkage from baseline is shown in Fig. 1A. Thirteen patients achieved pathological complete response (pCR), and the pCR rate was significantly higher than that of the null hypothesis (38.2% vs. 15%, P = 0.001). Twenty-seven patients (79.4%) had MPR. Pathological T stages after surgery were ypT0, ypT1b, ypT2, ypT3, and ypT4a in 13 (38.2%), 2 (5.9%), 9 (26.5%), 8 (23.5%), and 2 (5.9%) patients, respectively. Based on pathological N stage after surgery, 24 (70.6%), 2 (5.9%), 3 (8.8%), and 5 (14.7%) patients were ypN0, ypN1, ypN2, and ypN3, respectively (Table 2). The subgroup analysis of pCR is presented in Supplementary Fig. 2. Among the six participants with Bormann IV tumors, the MPR was 83.3% (5/6), the pCR rate was 16.7% (1/6), the rate of R0 resection was 100% (6/6), one was ypT0, one was ypT2, four were ypT3, three were ypN0, two were ypN2, and one was ypN3a.
Fig. 1. Treatment response (n = 34).
Tumor shrinkage from baseline (A) and duration of disease response (B). All patients had R0 resection. The duration of response was censored at the time of the procedure.
Table 2.
Pathologic responses after treatment
| Tumor response | Total (n = 34) | |
|---|---|---|
| n (%) | Confidence intervala (95%) | |
| R0 resection | 34 (100.0) | 0.90–1.00 |
| pCR | 13 (38.2) | 0.24–0.54b |
| MPR | 27 (79.4) | 0.62–0.91 |
| Pathological T stage post-surgery | ||
| ypT0 | 13 (38.2) | 0.22–0.56 |
| ypT1b | 2 (5.9) | 0.01–0.20 |
| ypT2 | 9 (26.5) | 0.13–0.44 |
| ypT3 | 8 (23.5) | 0.11–0.41 |
| ypT4a | 2 (5.9) | 0.01–0.20 |
| Pathological N stage post-surgery | ||
| ypN0 | 24 (70.6) | 0.53–0.85 |
| ypN1 | 2 (5.9) | 0.10–0.20 |
| ypN2 | 3 (8.8) | 0.02–0.24 |
| ypN3 | 5 (14.7) | 0.05–0.31 |
pCR pathological complete response, MPR major pathological response.
aThe confidence intervals were estimated by the Clopper-Pearson exact method.
bThe alpha level for the confidence interval of pCR was 90%.
Survival outcomes
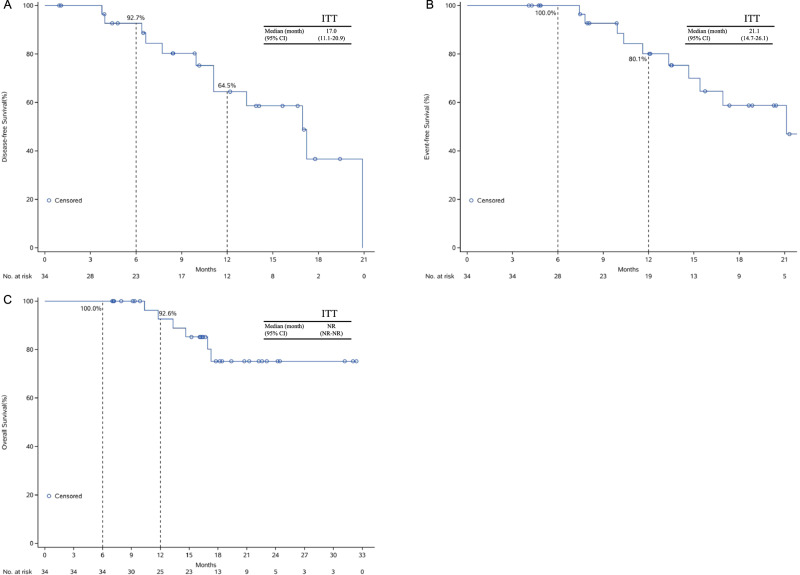
At the cutoff date of April 8, 2022, median follow-up times were 13.9 (range 1.0–20.9) months for disease-free survival (DFS) and 18.2 (range, 7.0–32.4) months for event-free survival (EFS) and OS. The survival of patients with different treatment responses is summarized in Fig. 1B. The median DFS was 17.0 (95%CI: 11.1–20.9) months (Fig. 2A), with a 1-year DFS rate of 64.5% (95%CI: 30.4–85.1). Patients achieving pCR had significantly longer median DFS than those who did not achieve pCR (20.9 vs. 11.1 months, log-rank P = 0.0285, Supplementary Fig. 3A). The median EFS was 21.1 (95%CI: 14.7–26.1) months, and the 1-year EFS rate was 80.1% (95%CI: 40.4–94.7, Fig. 2B). Six patients died during the follow-up period. The median OS was not reached. The 6-month and 1-year OS rates were 100.0% (95%CI: 89.7–100.0%) and 92.6% (95%CI: 50.1–99.5%), respectively (Fig. 2C).
Fig. 2. Survival outcomes of all patients.
(A) Disease-free survival, (B) event-free survival and (C) overall survival. Source data are provided as a Source Data file.
Safety profile
During the neoadjuvant period, 32 (94.1%), 31 (91.2%), and 11 (32.4%) patients experienced treatment-emergent AEs (TEAEs), treatment-related AEs (TRAEs), immune-related AEs (irAEs), respectively (Table 3). The most common TEAEs during the neoadjuvant period were myelosuppression (n = 27, 79.4%), nausea/vomiting (n = 17, 50.0%), and rash (n = 9, 26.5%). Seventeen patients (50.0%) had grade ≥3 AEs. The most common grade ≥3 AE was myelosuppression (n = 11, 32.4%). cCRT-related adverse events occurred in 28 (82.4%) patients, including 10 (29.4%) with grade 3 cCRT-related AEs (7 myelosuppression, 4 nausea/vomiting and 1 with both.) and 4 (11.8%) with grade 4 myelosuppression. The most common cCRT-related AEs were myelosuppression and nausea/vomiting (Supplementary Table 1). Two (5.9%) had radiotherapy discontinuation: one patient due to grade 3 nausea/vomiting at the dose of 36Gy/20f and one due to grade 3 nausea/vomiting and myelosuppression at the dose of 34.2 Gy/19f. The immunotherapy-related AEs (irAEs) are presented in Supplementary Table 2.
Table 3.
Safety profiles
| Adverse events, n (%) | Any periods (n = 34) | Neoadjuvant period (n = 34) | Surgery (n = 34) | Adjuvant period (n = 31) |
|---|---|---|---|---|
| TEAE | 34 (100.0) | 32 (94.1) | 13 (38.2) | 23 (74.2) |
| TRAE | 34 (100.0) | 31 (91.2) | 13 (38.2) | 22 (71.0) |
| irAE | 11 (32.4) | 11 (32.4) | 0 | 1 (3.2) |
| Grade ≥3 AEs | 22 (64.7) | 17 (50.0) | 1 (2.9) | 11 (35.5) |
| TEAEs | ||||
| Myelosuppression | 33 (97.1) | 27 (79.4) | 0 | 22 (71.0) |
| Nausea/vomiting | 18 (52.9) | 17 (50.0) | 0 | 1 (3.2) |
| Elevated ALT/AST | 11 (32.4) | 5 (14.7) | 5 (14.7) | 2 (6.5) |
| Decreased albumin | 9 (26.5) | 5 (14.7) | 3 (8.8) | 1 (3.2) |
| Rash | 9 (26.5) | 9 (26.5) | 0 | 0 |
| Hypokalemia | 8 (23.5) | 1 (2.9) | 6 (17.7) | 3 (9.7) |
| Fever | 3 (8.8) | 3 (8.8) | 0 | 0 |
| Fatigue | 2 (5.9) | 2 (5.9) | 0 | 0 |
| Pneumonia/pneumonitis | 2 (5.9) | 1 (2.9) | 1 (2.9) | 0 |
| Upper respiratory tract infection | 1 (2.9) | 1 (2.9) | 0 | 0 |
| Hypopituitarism | 1 (2.9) | 1 (2.9) | 0 | 1 (3.2) |
| Elevated bilirubin | 1 (2.9) | 1 (2.9) | 0 | 0 |
| Elevated creatinine | 1 (2.9) | 1 (2.9) | 0 | 0 |
| Hyperthyroidism | 1 (2.9) | 1 (2.9) | 0 | 0 |
| Urinary tract infection | 1 (2.9) | 1 (2.9) | 0 | 0 |
| Upper gastrointestinal bleeding | 1 (2.9) | 1 (2.9) | 0 | 0 |
| Anastomotic leakage | 1 (2.9) | 0 | 1 (2.9) | 0 |
| Intestinal obstruction | 1 (2.9) | 0 | 1 (2.9) | 0 |
AEs not indicated in this table had an occurrence of 0.
AE adverse event, TEAE treatment emergent adverse event, TRAE treatment related adverse event, irAE immune-related adverse event, ALT alanine aminotransferase, AST aspartate aminotransferase.
No patient postponed surgery due to AEs (Table 3). Thirteen patients (38.2%) experienced surgical AEs, and 1 (2.9%) had grade 3 surgical AEs. The most common surgical AEs were hypokalemia (n = 6, 17.7%) and elevated alanine aminotransferase/aspartate aminotransferase (n = 5, 14.7%). One (2.9%) patient developed a grade 2 operative complication of anastomotic leakage (Supplementary Table 3). During the adjuvant period, 23 patients (74.2%) had TEAEs, and most of them showed myelosuppression (n = 22, 71.0%). Eleven patients (35.5%) had grade ≥3 AEs, all of which were myelosuppression (Table 3).
Biomarker analysis
Baseline tumor biopsies were available in 94.1% (32/34) of patients. Patients with positive PD-L1 expression showed a numerically higher pCR rate (combined proportional score (CPS) ≥ 5 vs. CPS < 5, 63.6% vs. 28.6%, P = 0.072, Supplementary Fig. 2), while no association was found between PD-L1 expression and DFS (Supplementary Fig. 3B, C). One case was microsatellite-instability (MSI)-H and achieved pCR (Supplementary Fig. 2).
To further assess whether different immune cell subtypes within the TiME could predict treatment response, both baseline tumor biopsies and post-treatment surgical tissues were analyzed by the mIF assay. A total of 47 samples passed quality control and were tested in the final data analysis (23 who underwent gastroscopy and 24 who underwent surgery, including 17 samples that were paired).
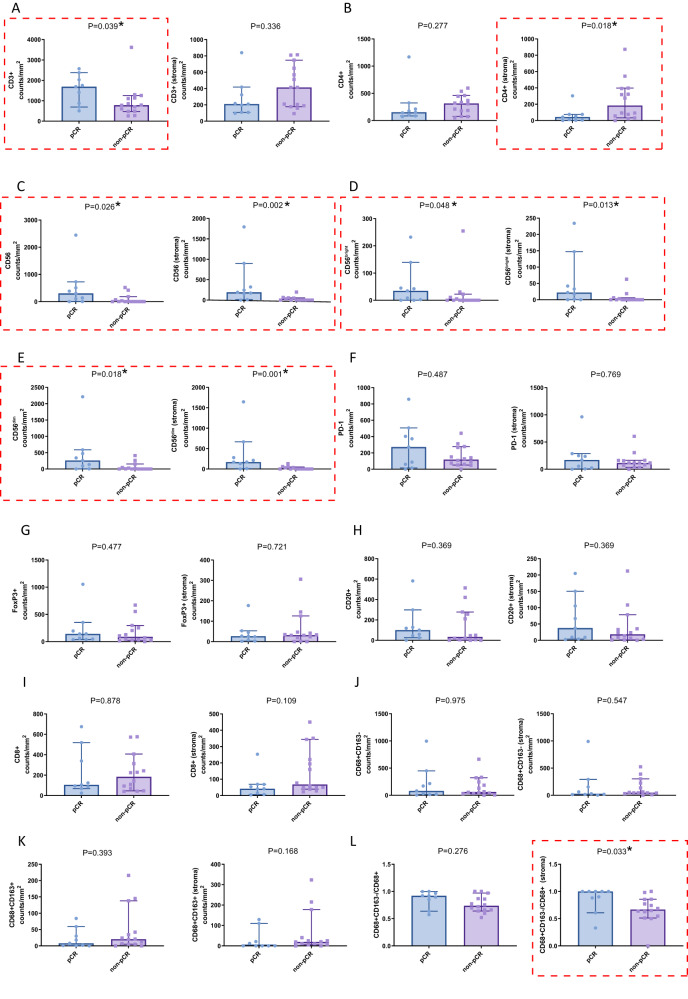
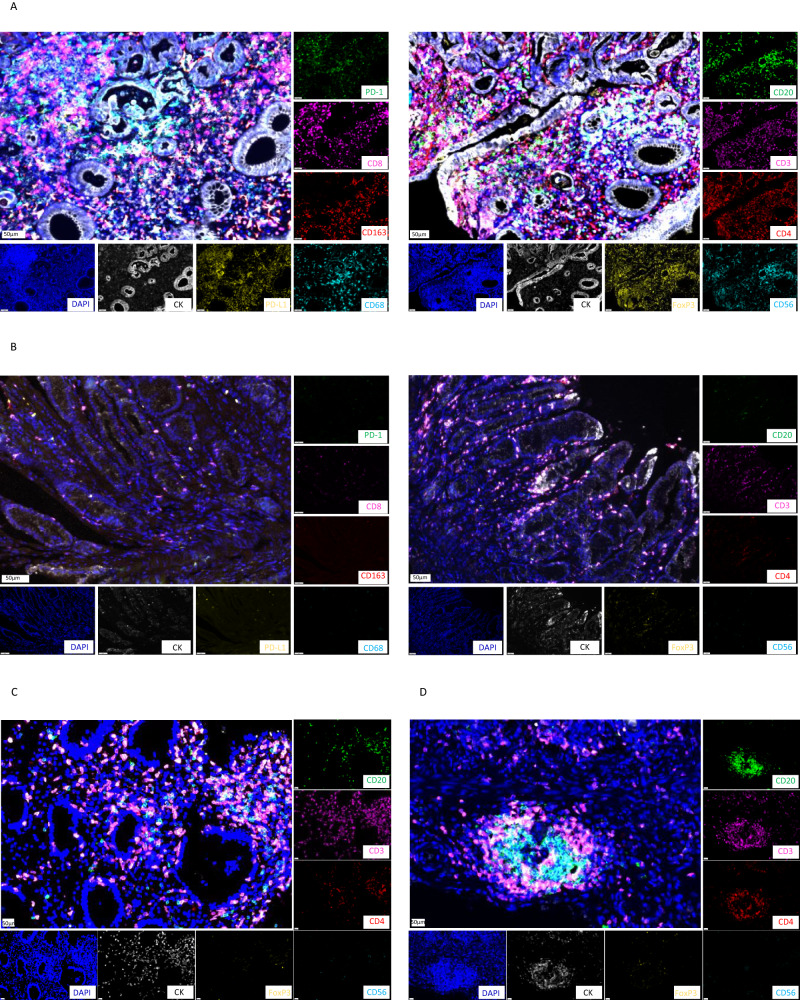
Patients achieving pCR had higher levels of infiltrating CD3+ T cells, CD4+ T cells, and CD56+ natural killers (NKs) (CD56bright and CD56dim subtypes) than those not achieving pCR (Fig. 3A–E). There were no significant differences in the levels of infiltrating CD20+ and CD8+ T cells and PD-1 and FoxP3 expression between the two groups (Fig. 3F–I). A significantly higher M1 to M1 + M2 macrophage infiltration ratio was observed in patients achieving pCR (Fig. 3J–L). After adjusting by Bonferroni correction test, the levels of CD56+ and CD56dim cells in stroma were positively correlated with efficacy with an adjusted P-value of 0.024 and 0.012, respectively, while adjusted P-values of other biomarkers were all >0.05 (Supplementary Table 4). Typical mIF images showed higher immune infiltration rates of CD56bright, CD56dim, and CD3 in two patients achieving pCR (Fig. 4A) compared with those not achieving pCR (Fig. 4B).
Fig. 3. Immune cell infiltration levels in the tumor tissue before neoadjuvant sintilimab and concurrent chemoradiotherapy between patients achieving pCR (n = 9) and those not achieving pCR (non-pCR; n = 14) assessed by multiplex immunofluorescence (mIF).
A–L The comparison of immune cell infiltration levels was performed using Friedman’s non-parametric test and the adjusted P-value using Bonferroni methods were presented in Supplementary Table 4. The error bars represented the standard deviation. Indicators marked with red dashed boxes represent p < 0.05. Source data are provided as a Source Data file.
Fig. 4. Representative multiplex immunofluorescence (mIF) of tumor immune microenvironment (TiME).
A, B Typical mIF images showing elevated CD56bright, CD56dim and CD3 cell infiltration in a patient achieving pCR (A) than another patient not achieving pCR (B). C, D Typical mIF images in a patient achieving pCR with relatively high levels of CD20+ B and CD3+ T cell infiltration at baseline (C) and developed tertiary lymphoid structure after therapy (D). The images are representative of three patients, bar = 50 um.
After neoadjuvant treatment, enhanced CD20+ B cell infiltration was found in patients achieving pCR than in patients not achieving pCR. Besides, a trend that infiltration of CD4+ T cells was higher in patients achieving pCR than that in patients not achieving pCR (median density: 10.1 vs. 0.4 counts/mm2, P = 0.059, adjusted P = 0.708) was observed. At the same time, there was no obvious significant difference in other biomarkers between the two groups (Supplementary Fig. 4). It should be noted that at baseline, two patients had relatively high levels of CD20+ B and CD3+ T cell infiltration, but TLSs were not detected, while TLSs were detected along with pCR after treatment (Fig. 4C, D). The changes in biomarkers are shown in Supplementary Fig. 5.
Discussion
The role of immunotherapy plus cCRT in the perioperative treatment of G/GEJ cancers is unknown. In this study, we evaluated the efficacy and safety of neoadjuvant sintilimab and cCRT followed by gastrectomy and adjuvant sintilimab and chemotherapy in patients with locally advanced G/GEJ cancers. Our results met the pre-specified primary endpoint, with a pCR rate of 38.2%. A total of 79.4% of patients achieved MPR, and all patients received R0 resection. The median DFS and EFS were 17.0 months and 21.1 months, respectively. In addition, biomarker analysis showed that the level of CD56+ and CD56dim NK cells in stroma at baseline were associated with pathological response.
In the neoadjuvant setting, the CROSS trial reported a pCR rate of 23% specifically in patients diagnosed with esophagus or GEJ adenocarcinoma, while the POET trial reported a pCR rate of 15.6% in patients with esophagus or GEJ cancer8,32. The interim results of the TOPGEAR trial suggest that preoperative radiation and intensive chemotherapy (epirubicin, cisplatin and fluorouracil) is safe for the vast majority of patients without additional treatment toxicity or surgical morbidity33. In addition to cCRT, several studies have investigated the role of preoperative immunotherapy-containing therapies in G/GEJ cancer patients. Neoadjuvant nivolumab monotherapy in resectable gastric cancers showed a pCR of only 3.2% and an MPR of 16.7%34. A phase II trial investigated preoperative pembrolizumab plus cCRT in 31 patients with GEJ cancer, of whom 7 (22.6%) achieved pCR, which did not meet the pre-specified primary endpoint35. Despite these two trials showing suboptimal efficacy, they demonstrated the potential effects of immunotherapy in the neoadjuvant setting for G/GEJ cancers. Likewise, another two single-arm phase II trials36,37 evaluated the effects of neoadjuvant PD-1 blockade plus chemotherapy or chemoradiotherapy in patients with locally advanced G/GEJ cancers, which showed promising efficacy, with a pCR rate of 28.0% and 33.3%, respectively. The ongoing phase II GASPAR trial of the perioperative FLOT regimen combined with spartalizumab will provide additional data in the future38. In addition, the benefit and risk of intensive chemotherapy regimen would be properly discussed when the full results of TOPGEAR study and other ongoing clinical trials that use the combination of FLOT and immunotherapy (e.g. MATTERHORN study, NCT04592913; KEYNOTE-585 study, NCT03221426).
In this study, 13 patients had pCR, whose rate was significantly higher than the null hypothesis (38.2% vs. 15.0%, P = 0.001), as well as higher than those of previous studies34–37. Better outcomes may be conferred by the combination of cCRT and immunotherapy. According to preclinical studies, cCRT enhances the immune response of the host and inhibits the immune escape of cancer cells during immunotherapy20,21. This combination was shown to improve the survival of cancer patients in the CheckMate-577 studies, in which cCRT was followed by subsequent immunotherapy22. Synergism with PD-1/PD-L1 blockade and radiotherapy was also reported in lung cancer with advanced disease stages39,40 and early-stage diseases41. Besides, the chemotherapy regimen in this study was nab-PTX, which showed promising antitumor activity in the first-line treatment of advanced gastric cancer30. As a potential immunomodulator, nab-PTX reverses the immunosuppressive microenvironment and promotes the cancer-immunity cycle in gastric cancer42, which was demonstrated to be effective in combination with concurrent radiotherapy or immunotherapy in various solid tumors43,44. Nevertheless, despite these potential reasons as well as the designed induction phase of immunotherapy plus chemotherapy in this study, the relatively high pCR rate among patients with advanced clinical tumor T stage (T3, 29.4%; T4, 71.7%) and a high proportion of lymph node metastasis (N2-3, 97.1%) demonstrated the encouraging efficacy of sintilimab in combination with cCRT for locally advanced G/GEJ adenocarcinomas, which could be further verified in future studies.
PD-L1 CPS was proven to predict outcomes in metastatic gastroesophageal cancer10; however, its predictive value was not determined in early-stage diseases. We found that patients with higher PD-L1 CPS (≥5 vs. CPS < 5) achieved a numerically higher pCR rate (63.6% vs. 28.6%, P = 0.072), consistent with a previous report35. However, PD-L1 CPS was not associated with survival outcomes (DFS & OS) in this study. This may be due to a relatively short follow-up time and immature survival data. Patients with MSI-H were reported to benefit from immunotherapy in colorectal and gastric cancers. In this study, one patient was MSI-H and achieved pCR, in line with previous studies45,46, indicating the potential predictive value of MSI-H, although limited by the sample size.
NK cells play a crucial role in early antitumor immunity by directly killing tumor cells47. CD56dim NK cells are the final mature stage of NK cells and have a higher killing ability compared with CD56bright NK cells. Studies have shown higher levels of NK cell infiltration are associated with better tumor outcomes48. A better therapeutic response was associated with both CD56dim and CD56bright NK cells in this study. As a result, CD56+ NK cells might be a good predictor of the response to immunotherapy plus cCRT in locally advanced G/GEJ cancers. Tumor-associated macrophages play important roles in tumor immune response, tumor cell proliferation, and tumor invasion49. Immune responses can be induced by the transformation of type M2 (pro-tumor type) macrophages into type M1 (antitumor type) macrophages49. In this study, patients achieving pCR showed significantly higher M1 to M1 + M2 macrophage infiltration ratio, which was consistent with a previous study50. CD20 is a receptor on B lymphocytes, which play an important role in the regulation of the immune system and antitumor activity51. The infiltration of CD20+ B cells was enhanced in patients achieving pCR in this study, in agreement with a previous study52. Additionally, CD20+ B cells are a strong prognostic factor, and patients with abundant B cells have longer survival after treatment with immune checkpoint inhibitors53, which was verified by long-term follow-up in this study.
A TLS is an ectopic lymphoid organ induced by chronic inflammation and tumors, mainly composed of CD20+ B cells and CD3+ T cells, which has been associated with better outcomes following treatment with immune checkpoint inhibitors54. Studies have shown that chemotherapy and immunotherapy induce TLS formation and B cell aggregation55,56. In this study, two patients had TLSs after immunotherapy plus cCRT and achieved pCR, which is consistent with a previous study revealing elevated response rate to melanoma neoadjuvant immunotherapy is associated with TLS detection in on-treatment samples52. Among the patient with TLS at baseline, the pCR rate was 40% (2/5), similar to 38.2% in the whole cohort. The MPR rate was 80%, also similar to 79.2% in the whole cohort. It is possible that endoscopy biopsy does not cover large tumor stromal areas rich in TLS structure, and the number of TLSs will be underestimated57. According to these findings, immunotherapy combined with cCRT for locally advanced G/GEJ cancers modulates the TiME, including triggering TLS generation, which might be associated with improved survival and response.
Regarding survival, the 6-month and 1-year OS rates in this study were 100.0% and 92.6%, respectively. One-year OS rates of 79.8% and 75% were reported for patients with GEJ cancers using neoadjuvant cCRT in the CROSS and POET studies, respectively8,32. Besides, we found that patients with pCR had longer median DFS than those with Non-pCR (20.9 vs. 11.1 months), consistent with a previous report32. Considering the relatively short median follow-up time (18.2 months, range: 7.0–32.4) and immature OS data, whether the improved pCR could translate to survival benefit in this trial should be further investigated.
The most common TEAE during the neoadjuvant period in this study was myelosuppression, followed by nausea/vomiting and rash, corroborating previous studies36. A total of 17 patients experienced manageable grade ≥3 AEs, of which the most common was myelosuppression. One patient developed a grade II operative complication of anastomotic leakage due to improper eating, which was also reported in a previous study in which anastomotic leakage occurred in 10.3% (3/29) of patients35. The case in this study recovered seven days later upon fasting and symptomatic treatment and did not undergo a second operation. There was no additional safety concern, suggesting the feasibility of sintilimab in combination with cCRT.
Nab-paclitaxel was selected in the present study for several reasons. First, there is evidence of the efficacy of paclitaxel in gastric cancer25,58–60 and nab-paclitaxel in the second- and third-line treatment of gastric cancer26–28. Second, there is also evidence of the non-inferior efficacy of nab-paclitaxel vs. paclitaxel in advanced gastric cancer29. In Japan, nab-paclitaxel is approved for the second-line treatment of gastric cancer26,27. Nab-paclitaxel has been shown effective as a first-line treatment28 and demonstrated longer progression-free survival than paclitaxel in first-line treatment for advanced gastric cancer30. Third, nab-paclitaxel has a synergistic effect with immunotherapy61,62. In addition, nab-paclitaxel has a synergistic effect with radiotherapy in lung cancer, pancreatic cancer, and head and neck squamous cancer, among others43,63–65. Fourth, nab-paclitaxel does not require premedication with corticosteroids, which might be more suitable in the context of the unsure impact of corticosteroids in patients receiving immunotherapy. Although there is no compelling evidence that corticosteroids decrease the efficacy of immunotherapy66, corticosteroids and immunotherapy both influence the immune system in different ways67, and it was considered prudent when designing the trial to avoid that potential confounder.
In the present study, radiotherapy was given according to local physicians’ preferences, according to guidelines, experience, and tumor board discussions. Future studies could look into specific radiotherapy regimens for preoperative sintilimab in combination with cCRT. Different doses and fractionation regimens could yield different results and should be explored in the future.
This study had some limitations. This was a single-arm trial with a limited sample size, and no control group was included. Although this study showed promising short-term outcomes (e.g., pCR rate) and middle-term outcomes (e.g., DFS and EFS), the long-term efficacy of the combination perioperative therapy was not assessed. A follow-up study is ongoing, and survival data will be reported in the future. This study was a preliminary exploratory phase II trial, and selecting the pCR as the primary endpoint is common in such trials of solid tumors as it provides an answer more rapidly than survival68–70. In the RTOG 9904 trial, the patients with pCR achieved better outcomes than the non-pCR patients, and pCR was suggested as a prognostic surrogate69,71. Future studies will have a longer follow-up. Only one case of grade 2 fatigue was reported in our study, a finding which seems to diverge from expectations. The relatively small sample size may have led to an incomplete capture of patient experiences. Additionally, the potential for less stringent reporting and observation practices of adverse reactions such as fatigue could have been a contributing factor. Fatigue is inherently subjective, and thus its accurate assessment can be challenging. Finally, this study explored biomarkers, but the sample size does not allow for conclusions. The biomarker results could be referred to for the design of future large-scale trials.
In this multicenter, single-arm trial, sintilimab in combination with cCRT demonstrated promising efficacy and a favorable safety profile in the perioperative setting for locally advanced G/GEJ adenocarcinomas. Further large-scale randomized clinical trials are warranted to confirm the survival benefit.
Methods
Study design and participants
The SHARED study (ChiCTR1900024428) followed the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the ethics committees of the Comprehensive Cancer Centre of Drum Tower Hospital (2019-093-02) and other participating centers. All patients provided written informed consent before any procedure.
This study was a multicenter, single-arm phase 2 trial conducted at three centers in China (The date of registration was July 11, 2019, https://www.chictr.org.cn/showprojEN.html?proj=40332). The study protocol was published previously72. Treatment-naïve patients aged over 18, with histologically or cytologically confirmed locally advanced G/GEJ cancers (cT3N2-3M0, cT4aN + M0, or cT4bNanyM0, determined by computed tomography and/or magnetic resonance imaging before treatments), an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0-1, and at least one measurable lesion based on Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 were enrolled from July 20th 2019 to October 10th 2021.
Patients with gastric neuroendocrine tumors, distant metastasis, uncontrolled pleural effusion, pericardial effusion, ascites, history of chemotherapy, radiotherapy, or immunotherapy, history of cancer within the past 5 years (except basal cell or squamous cell skin cancer, superficial bladder cancer, in-situ cervical cancer, or in-situ breast cancer), gastrointestinal obstruction, uncontrolled infection, uncontrolled systemic disease, or the use of immunosuppressive agents or experimental drugs in the past 4 weeks were excluded. Patients with severe cardiovascular diseases, such as symptomatic coronary heart disease, grade II congestive heart failure, uncontrolled arrhythmia and myocardial infarction, occurred within 12 months before admission; or with a history of interstitial pulmonary disease, non-infectious pneumonitis, pulmonary fibrosis, and acute pulmonary disease; or respiratory condition that required any oxygen supplementation, active pneumonitis and clinically significant pulmonary hypertension occurred within 12 months before admission were excluded. Pregnant, breastfeeding, or pregnancy test-positive women were also excluded72.
Procedure
Eligible patients received one 3-week cycle of induction treatment consisting of S-1 (40 mg/m2, PO, bid, D1-14), nab-paclitaxel (nab-PTX, 100–120 mg/m2, IV, D1, and 8), and sintilimab (200 mg, IV, D1), followed by 5 weeks of radiation therapy (45 Gy/1.8 Gy in 25 factions), nab-PTX (80–100 mg/m2, IV, D1, 8, 15, and 22) and sintilimab (200 mg, IV, D1, 22). Patients were administered another 3-week cycle of S-1 (40 mg/m2, PO, bid, D1-14), nab-PTX (100–120 mg/m2, IV, D1 and 8) plus sintilimab (200 mg, IV, D1) one to three weeks later, and surgery was preferably scheduled within 1-3 weeks. Four to six weeks after surgery, three 3-week cycles of S-1 (40 mg/m2, PO, bid, D1-14), nab-PTX (100–120 mg/m2, IV, D1 and 8) plus sintilimab (200 mg, IV, D1) were administered as adjuvant therapy72. Dose modification of sintilimab was not permitted, and treatment was discontinued in case of disease progression, death, or unacceptable toxicities. Patients experiencing grade IV leukopenia with fever were administered prophylactic anti-infective therapy with broad-spectrum antibiotics.
Prior to radiotherapy, computed tomography (CT) simulation set as axial scanning with a layer thickness of 3 mm, complemented with abdominal compression, four-dimensional CT, and respiratory gating technologies, was performed to manage respiratory movement and accurately localize the target area. The gross tumor volume (GTV) included primary tumors and metastatic lymph nodes. The clinical target volume (CTV) included GTV and high-risk lymphatic drainage area. The planed target volume (PTV) included CTV with a 5-mm expansion in all direction. The radiotherapy treatment plan included a total dose of 45 Gy, typically administered in 25 fractions over five weeks. Radiotherapy was delivered using conformal, intensity-modulated, or spiral tomographic intensity-modulated techniques, with photon radiation typically at an energy level of 6 MV or 8 MV for conformal radiotherapy and 6 MV for intensity-modulated radiotherapy. The radiotherapy process was meticulously overseen by a multidisciplinary quality assurance team.
A multidisciplinary team assessed the patients’ conditions and decided on the surgical protocol according to guidelines5,73, experience, and tumor board discussions. The technical details of surgery and radiotherapy were presented as Supplementary Note in the Supplementary information file.
Tumor assessments were evaluated per RECIST v1.1 by CT/magnetic resonance imaging (MRI) and endoscopic ultrasound (EUS) every six weeks preoperatively, every nine weeks postoperatively, and every three months after treatment completion until disease progression (up to two years) or new anticancer treatment initiation. Routine blood tests and blood biochemistry were reviewed weekly. Tumor biomarkers and thyroid function were reviewed preoperatively at weeks 3 and 8 of neoadjuvant therapy and postoperatively every 3 weeks. Adverse events (AEs) from the first dosing of the study regimen to 90 days after the last dosing were recorded and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI CTCAE, version 4.03). Surgical complications were grading using Clavien-Dindo. Judgment and grading of the AEs were based on the time of occurrence of the AEs, clinical manifestations, and relevant test and examination results. If necessary, multidisciplinary discussions were held with relevant departments such as imaging, immunology, gastroenterology, and respiratory.
Endpoints
The primary endpoint was the pCR rate, i.e., the absence of viable residual tumor cells in the resected specimen. Secondary endpoints included major pathological response (MPR, i.e., residual tumor cells below 10% in the resected specimen), R0 resection rate (i.e., complete removal of the tumor with a tumor-free margin), DFS, EFS, and OS. DFS was defined as the time from surgery to postoperative recurrence or death from any cause, whichever occurred first. DFS was censored on the last tumor assessment date for patients still alive and without recurrence. EFS was the time from enrollment to recurrence or death from any cause. EFS was censored on the last tumor assessment date for patients still alive and without recurrence. OS was the time from enrolment to death from any cause. OS was censored on the last date known to be alive for patients without documentation of death. Safety endpoints included TEAEs, TRAEs, irAEs, and surgical AEs (i.e., complications occurring during or within 30 days of surgery).
Biomarker analysis
PD-L1 expression was assessed in formalin-fixed paraffin-embedded tumor samples before treatment by the PD-L1 immunohistochemistry (IHC) 22C3 pharmDx assay (Dako, Glostrup, Denmark). PD-L1 evaluation was performed using the CPS, determined as the number of PD-L1-positive cells—tumor cells, lymphocytes, and macrophages—divided by the total number of tumor cells × 100. PD-L1 positivity was defined as CPS ≥ 1 or 5. Mismatch repair (MMR) status was assessed locally by polymerase chain reaction (PCR) amplification and fragment analysis or IHC analysis of the DNA mismatch repair proteins MLH1, MSH2, MSH6, and PMS2. Monoclonal antibodies against MLH1 (Clone: ES05, dilution 1:100, Dako Denmark A/S, Denmark), PMS2 (Clone: EP51, dilution 1:100, Dako Denmark A/S, Denmark), MSH2 (Clone: FE11, dilution 1:100, Dako Denmark A/S, Denmark), MSH6 (Clone: EP49, dilution 1:150, Dako Denmark A/S, Denmark) were incubated with tumor sections in a humidified chamber at 4 °C overnight. Negative controls (without the primary antibody) and positive controls were included in each run to ensure the specificity and accuracy of the staining procedure. MSI analysis was performed using the Revised Bethesda Guidelines for two mononucleotides (BAT 25 and BAT 26) and 3 dinucleotides (D2S123, D5S346, and D17S250) microsatellite markers. Tumors were classified as MSI-H/dMMR with 2 or more unstable markers74 or with no expression of MMR proteins.
The biomarker panel was decided by the trial committee through discussion when designing the trial based on guidelines5,6 and experience. This panel of 10 biomarkers was selected based on the efficacy of immunotherapy in solid tumors, including biomarkers for T cells (CD3, CD4, and CD8)75,76, Tregs (FoxP3)77, B cells (CD20)52, NK cells (CD56)47, macrophages (CD68, CD163)78, and immune escape (PD-1/L1)46. The TiME was examined on baseline tumor biopsies and surgical specimens. The Akoya OPAL Polaris Seven-Color Automation multiplex immunohistochemistry panels were applied for mIF staining following the manufacturer’s instructions. Primary antibodies used were raised against CD163 (Abcam, ab182422, 1:500), CD8 (Abcam, ab178089, 1:200), CD68 (Abcam, ab213363, 1:1000), CD20 (DAKO, L26, IR604, 1:1), CD3 (DAKO, A0452, 1:1), CD4 (Abcam, ab133616, 1:100), CD56 (Abcam, ab75813, 1:1000), and FoxP3 (Abcam, ab20034, 1:100) for panel 2. Nucleic acids were stained with DAPI. Tumor parenchyma and stroma were differentiated according to pan-CK staining (Abcam, ab7753, 1:100). Digital image analysis was performed with the APTIME image analysis software (3D Medicines). Tertiary lymphoid structures (TLSs) were defined as CD20+ B cell aggregates surrounded by accumulated CD3+ T cells. The density of various immune cell subsets was expressed as the count of positively stained cells per square millimeter (cells/mm2). The total density was calculated by dividing the count of tumor and stroma cells by the area of the tumor and stroma.
Statistical analysis
A Simon 2-stage design was employed. The null hypothesis was a pCR rate of 15% was determined based on our clinical experience and the pCR rate of 16% reported in the FLOT4-AIO trial79, as chemotherapy was the standard of care. The alternative hypothesis was a pCR rate of ≥35% in this study. Using a power of 80% and α = 0.05, a minimum of nine patients were needed in stage I, and if a pCR was confirmed in one or two of them, an additional 25 patients were enrolled in stage II. The analysis plan was designed before conducting the trial. Descriptive statistics were primarily used. Continuous data were described as median (range), and categorical data were described as frequency (percentage). The primary endpoint of pCR with a 90% confidence interval (CI) in the whole population and 95% CIs in subgroups were estimated using the Clopper-Pearson method. A binomial test was performed for pCR with the null hypothesis of pCR as 0.15. For MPR, R0 resection, pathological T stages, and pathological N stages, the 95% CIs were estimated by the Clopper-Pearson exact method without statistical test. Time-to-event variables (DFS, EFS, and OS) were analyzed by the Kaplan-Meier method and compared by the Log-rank test. The hazard ratio (HR) and 95% CIs were estimated using the Cox proportional hazards model. In the exploratory analysis of pathological response, associations of categorical variables were analyzed via Chi-square or Fisher Exact test. Continuous data between groups of pathological response were compared by the Mann–Whitney U test. Bonferroni correction test was used for multiplicity corrections in biomarker analysis. All statistical analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Two-sided P < 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
The authors thank the participating patients and their families and the study centers and investigators for their contributions to the study. The authors also thank Mengmeng Tang, Xin Zhang, Yue Wang and Yipeng Zhang for their assistance in data collection and sample checking. This trial was supported by Funding for Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University (2022-YXZX-ZL-01) and Beijing Xisike Clinical Oncology Research Foundation (Y-Young2023-019). All investigators received no remuneration. Innovent Biologics (Suzhou) Co., Ltd. provided sintilimab but had no role in the study design or writing of the manuscript.
Author contributions
B.R.L., W.X.G., and J.W. conceived and designed the study. B.R.L., W.X.G., J.W., and H.T.Y. were responsible for patient recruitment. Q.L., J. Yang, Y.Y., C.Z., and N.Q.D. were responsible for patient treatment and patient care. X.F.L., M.W., L.M.Z., and P.Z. were responsible for surgery. X.S.F., Y.F., L.L., and F.C.L. helped with pathological evaluation. S.L. helped with image evaluation. J. Yan was responsible for quality control of radiotherapy. X.Y.Z. collected the data. Y.Z. and J.W.Z. analyzed and interpreted clinical data. H.C., S.Q.C., and X.C.Z. were responsible for biomarker analysis. JW wrote the first draft of the manuscript.
Peer review
Peer review information
Nature Communications thanks Stephanie Daignault-Newton and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
Patient baseline clinical data are available in Table 1 and within the text. The surgery protocol and radiotherapy protocol are available as Supplementary Note in the Supplementary information file. Study Protocol is available in Ref. 72. The individual de-identified participant data, Statistical Analysis plan and the full image dataset are available for scientific purpose by sending requests to the corresponding author Baorui Liu (baoruiliu@nju.edu.cn) within 5 years after this paper’s publication. The remaining data are available within the Article, Supplementary Information, or Source Data file. Source data are provided with this paper.
Competing interests
H.C., S.C., and X.Z. are employees of 3D Medicines Inc. (Shanghai, China). The remaining authors have no conflicts of interest to declare.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wenxian Guan, Email: guan-wx@163.com.
Baorui Liu, Email: baoruiliu@nju.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-40480-x.
References
- 1.Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Zheng R, et al. Cancer incidence and mortality in China, 2016. J. Natl Cancer Center. 2022;2:1–9. doi: 10.1016/j.jncc.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng H, et al. Disparities in stage at diagnosis for five common cancers in China: a multicentre, hospital-based, observational study. Lancet Public Health. 2021;6:e877–e887. doi: 10.1016/S2468-2667(21)00157-2. [DOI] [PubMed] [Google Scholar]
- 4.Petrelli F, et al. Neoadjuvant chemoradiotherapy or chemotherapy for gastroesophageal junction adenocarcinoma: a systematic review and meta-analysis. Gastric Cancer. 2019;22:245–254. doi: 10.1007/s10120-018-0901-3. [DOI] [PubMed] [Google Scholar]
- 5.Wang F-H, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. (Lond) 2021;41:747–795. doi: 10.1002/cac2.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ajani JA, et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl Compr. Canc. Netw. 2022;20:167–192. doi: 10.6004/jnccn.2022.0008. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22:1081–1092. doi: 10.1016/S1470-2045(21)00297-7. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro J, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 9.Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021;71:264–279. doi: 10.3322/caac.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janjigian YY, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen L, et al. 1445P - CS1001, an anti-PD-L1 antibody, combined with standard of care (SOC) chemotherapy for first line (1L) advanced GC/GEJ and ESCC: Preliminary results from 2 phase Ib cohorts of CS1001-101 study. Ann. Oncol. 2020;31:S841–S873. [Google Scholar]
- 12.Xu J, et al. LBA53 Sintilimab plus chemotherapy (chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): first results of a randomized, double-blind, phase III study. Ann. Oncol. 2021;32:S1331. [Google Scholar]
- 13.Versluis JM, Long GV, Blank CU. Learning from clinical trials of neoadjuvant checkpoint blockade. Nat Med. 2020;26:475–484. doi: 10.1038/s41591-020-0829-0. [DOI] [PubMed] [Google Scholar]
- 14.Loibl, S. et al. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann. Oncol.10.1016/j.annonc.2022.07.1940 (2022). [DOI] [PubMed]
- 15.Xia, Y. et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: a single-arm, open label, phase II clinical trial. J. Immunother. Cancer10, 10.1136/jitc-2022-004656 (2022). [DOI] [PMC free article] [PubMed]
- 16.Forde PM, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378:1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blank CU, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24:1655–1661. doi: 10.1038/s41591-018-0198-0. [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin M, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer. 2020;20:203–217. doi: 10.1038/s41568-020-0246-1. [DOI] [PubMed] [Google Scholar]
- 19.Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021;221:107753. doi: 10.1016/j.pharmthera.2020.107753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golden EB, et al. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518. doi: 10.4161/onci.28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. The Lancet Oncology. 2015;16:e498–e509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 22.Kelly RJ, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N. Engl. J. Med. 2021;384:1191–1203. doi: 10.1056/NEJMoa2032125. [DOI] [PubMed] [Google Scholar]
- 23.Li S, et al. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat. Commun. 2023;14:8. doi: 10.1038/s41467-022-35431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, et al. Durable blockade of PD−1 signaling links preclinical efficacy of sintilimab to its clinical benefit. MAbs. 2019;11:1443–1451. doi: 10.1080/19420862.2019.1654303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gadgeel SM, et al. Phase II study of paclitaxel and carboplatin in patients with advanced gastric cancer. Am. J. Clin. Oncol. 2003;26:37–41. doi: 10.1097/00000421-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki Y, et al. Phase II trial of nanoparticle albumin-bound paclitaxel as second-line chemotherapy for unresectable or recurrent gastric cancer. Cancer Sci. 2014;105:812–817. doi: 10.1111/cas.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuchi M, et al. Efficacy of nab-paclitaxel as second-line chemotherapy for unresectable or recurrent gastric cancer. Anticancer Res. 2016;36:6699–6703. doi: 10.21873/anticanres.11281. [DOI] [PubMed] [Google Scholar]
- 28.He MM, et al. Phase II clinical trial of S-1 plus nanoparticle albumin-bound paclitaxel in untreated patients with metastatic gastric cancer. Cancer Sci. 2018;109:3575–3582. doi: 10.1111/cas.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shitara K, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol. Hepatol. 2017;2:277–287. doi: 10.1016/S2468-1253(16)30219-9. [DOI] [PubMed] [Google Scholar]
- 30.Dai YH, et al. Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) Ther. Adv. Med. Oncol. 2022;14:17588359221118020. doi: 10.1177/17588359221118020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tokunaga M, et al. Perioperative chemotherapy for locally advanced gastric cancer in Japan: current and future perspectives. Surg. Today. 2020;50:30–37. doi: 10.1007/s00595-019-01896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stahl M, et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur. J. Cancer. 2017;81:183–190. doi: 10.1016/j.ejca.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 33.Leong T, et al. TOPGEAR: a randomized, phase III trial of perioperative ECF chemotherapy with or without preoperative chemoradiation for resectable gastric cancer: interim results from an international, intergroup Trial of the AGITG, TROG, EORTC and CCTG. Ann. Surg. Oncol. 2017;24:2252–2258. doi: 10.1245/s10434-017-5830-6. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa H, et al. A multicenter, open-label, single-arm phase I trial of neoadjuvant nivolumab monotherapy for resectable gastric cancer. Gastric Cancer. 2022;25:619–628. doi: 10.1007/s10120-022-01286-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu M, et al. Pembrolizumab in combination with neoadjuvant chemoradiotherapy for patients with resectable adenocarcinoma of the gastroesophageal junction. Clin Cancer Res. 2022;28:3021–3031. doi: 10.1158/1078-0432.CCR-22-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang X, et al. Neoadjuvant PD-1 blockade plus chemotherapy induces a high pathological complete response rate and anti-tumor immune subsets in clinical stage III gastric cancer. Oncoimmunology. 2022;11:2135819. doi: 10.1080/2162402X.2022.2135819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang Z, et al. The Neo-PLANET phase II trial of neoadjuvant camrelizumab plus concurrent chemoradiotherapy in locally advanced adenocarcinoma of stomach or gastroesophageal junction. Nat. Commun. 2022;13:6807. doi: 10.1038/s41467-022-34403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dos Santos, M. et al. Perioperative treatment in resectable gastric cancer with spartalizumab in combination with fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT): a phase II study (GASPAR). BMC Cancer22, 537 (2022). [DOI] [PMC free article] [PubMed]
- 39.Theelen W, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1276–1282. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Theelen WSME, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir. Med. 2021;9:467–475. doi: 10.1016/S2213-2600(20)30391-X. [DOI] [PubMed] [Google Scholar]
- 41.Altorki NK, et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol. 2021;22:824–835. doi: 10.1016/S1470-2045(21)00149-2. [DOI] [PubMed] [Google Scholar]
- 42.Zhu M, et al. Targeting ZFP64/GAL-1 axis promotes therapeutic effect of nab-paclitaxel and reverses immunosuppressive microenvironment in gastric cancer. J. Exp. Clin. Cancer Res. 2022;41:14. doi: 10.1186/s13046-021-02224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka H, et al. A phase I/II study of biweekly carboplatin and nab-paclitaxel with concurrent radiotherapy for patients with locally advanced unresectable stage iii non-small-cell lung cancer. Clin. Lung Cancer. 2021;22:42–48. doi: 10.1016/j.cllc.2020.09.016. [DOI] [PubMed] [Google Scholar]
- 44.Schmid P, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 45.Diaz LA, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022;23:659–670. doi: 10.1016/S1470-2045(22)00197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim ST, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 2018;24:1449–1458. doi: 10.1038/s41591-018-0101-z. [DOI] [PubMed] [Google Scholar]
- 47.Watkins-Schulz R, et al. A microparticle platform for STING-targeted immunotherapy enhances natural killer cell- and CD8(+) T cell-mediated anti-tumor immunity. Biomaterials. 2019;205:94–105. doi: 10.1016/j.biomaterials.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stangl S, et al. Heat shock protein 70 and tumor-infiltrating NK cells as prognostic indicators for patients with squamous cell carcinoma of the head and neck after radiochemotherapy: a multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG) Int. J. Cancer. 2018;142:1911–1925. doi: 10.1002/ijc.31213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romano E, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc. Natl Acad. Sci. USA. 2015;112:6140–6145. doi: 10.1073/pnas.1417320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pavlasova G, Mraz M. The regulation and function of CD20: an “enigma” of B-cell biology and targeted therapy. Haematologica. 2020;105:1494–1506. doi: 10.3324/haematol.2019.243543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helmink BA, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petitprez F, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577:556–560. doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- 54.Cabrita R, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:561–565. doi: 10.1038/s41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- 55.Lu Y, et al. Complement signals determine opposite effects of B cells in chemotherapy-induced immunity. Cell. 2020;180:1081–1097 e1024. doi: 10.1016/j.cell.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez AB, et al. Immune mechanisms orchestrate tertiary lymphoid structures in tumors via cancer-associated fibroblasts. Cell Rep. 2021;36:109422. doi: 10.1016/j.celrep.2021.109422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer. 2019;19:307–325. doi: 10.1038/s41568-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 58.Ilson DH, et al. A phase II trial of paclitaxel and cisplatin in patients with advanced carcinoma of the esophagus. Cancer J. 2000;6:316–323. [PubMed] [Google Scholar]
- 59.Ilson DH, Wadleigh RG, Leichman LP, Kelsen DP. Paclitaxel given by a weekly 1-h infusion in advanced esophageal cancer. Ann. Oncol. 2007;18:898–902. doi: 10.1093/annonc/mdm004. [DOI] [PubMed] [Google Scholar]
- 60.Petrasch S, et al. Chemotherapy with cisplatin and paclitaxel in patients with locally advanced, recurrent or metastatic oesophageal cancer. Br. J. Cancer. 1998;78:511–514. doi: 10.1038/bjc.1998.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen L, et al. Famitinib with camrelizumab and nab-paclitaxel for advanced immunomodulatory triple-negative breast cancer (FUTURE-C-Plus): an open-label, single-arm, phase II trial. Clin. Cancer Res. 2022;28:2807–2817. doi: 10.1158/1078-0432.CCR-21-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J, et al. Synergistic effects of nab-PTX and anti-PD-1 antibody combination against lung cancer by regulating the Pi3K/AKT pathway through the Serpinc1 gene. Front. Oncol. 2022;12:933646. doi: 10.3389/fonc.2022.933646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takano N, et al. Phase II study of chemoradiotherapy combined with gemcitabine plus nab-paclitaxel for unresectable locally advanced pancreatic ductal adenocarcinoma (NUPAT 05 Trial): study protocol for a single arm phase II study. Nagoya J. Med. Sci. 2019;81:233–239. doi: 10.18999/nagjms.81.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu K, et al. A phase II study of concurrent nab-paclitaxel/carboplatin combined with thoracic radiotherapy in locally advanced squamous cell lung cancer. J Thorac. Dis. 2019;11:4529–4537. doi: 10.21037/jtd.2019.10.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamada S, et al. Phase I study of chemoradiotherapy using gemcitabine plus nab-paclitaxel for unresectable locally advanced pancreatic cancer. Cancer Chemother. Pharmacol. 2018;81:815–821. doi: 10.1007/s00280-018-3554-3. [DOI] [PubMed] [Google Scholar]
- 66.Nelson, B. E. et al. Corticosteroid use and its impact on the efficacy of immunotherapy in multiple tumor types. J. Clin. Oncol.39, 10.1200/JCO.2021.39.15_suppl.e14583 (2021).
- 67.Aldea M, et al. How to manage patients with corticosteroids in oncology in the era of immunotherapy. Eur. J. Cancer. 2020;141:239–251. doi: 10.1016/j.ejca.2020.09.032. [DOI] [PubMed] [Google Scholar]
- 68.Jiang, H. et al. Efficacy and safety of neoadjuvant sintilimab, oxaliplatin and capecitabine in patients with locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma: early results of a phase 2 study. J. Immunother. Cancer10, 10.1136/jitc-2021-003635 (2022). [DOI] [PMC free article] [PubMed]
- 69.Ren S, et al. A narrative review of primary research endpoints of neoadjuvant therapy for lung cancer: past, present and future. Transl. Lung Cancer Res. 2021;10:3264–3275. doi: 10.21037/tlcr-21-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Conforti F, et al. Evaluation of pathological complete response as surrogate endpoint in neoadjuvant randomised clinical trials of early stage breast cancer: systematic review and meta-analysis. BMJ. 2021;375:e066381. doi: 10.1136/bmj-2021-066381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ajani JA, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J. Clin. Oncol. 2006;24:3953–3958. doi: 10.1200/JCO.2006.06.4840. [DOI] [PubMed] [Google Scholar]
- 72.Wei J, et al. Efficacy and safety of sintilimab in combination with concurrent chemoradiotherapy for locally advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma (SHARED): study protocol of a prospective, multi-center, single-arm phase 2 trial. Cancer Manag. Res. 2022;14:2007–2015. doi: 10.2147/CMAR.S355687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Japanese Gastric Cancer, A. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition) Gastric Cancer. 2023;26:1–25. doi: 10.1007/s10120-022-01331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim DG, et al. Clinical implications of microsatellite instability in early gastric cancer. J. Gastric Cancer. 2019;19:427–437. doi: 10.5230/jgc.2019.19.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tumeh PC, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosenberg JE, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kamada T, et al. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl Acad. Sci. USA. 2019;116:9999–10008. doi: 10.1073/pnas.1822001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hensler, M. et al. M2-like macrophages dictate clinically relevant immunosuppression in metastatic ovarian cancer. J. Immunother. Cancer8, 10.1136/jitc-2020-000979 (2020). [DOI] [PMC free article] [PubMed]
- 79.Al-Batran SE, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17:1697–1708. doi: 10.1016/S1470-2045(16)30531-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Patient baseline clinical data are available in Table 1 and within the text. The surgery protocol and radiotherapy protocol are available as Supplementary Note in the Supplementary information file. Study Protocol is available in Ref. 72. The individual de-identified participant data, Statistical Analysis plan and the full image dataset are available for scientific purpose by sending requests to the corresponding author Baorui Liu (baoruiliu@nju.edu.cn) within 5 years after this paper’s publication. The remaining data are available within the Article, Supplementary Information, or Source Data file. Source data are provided with this paper.