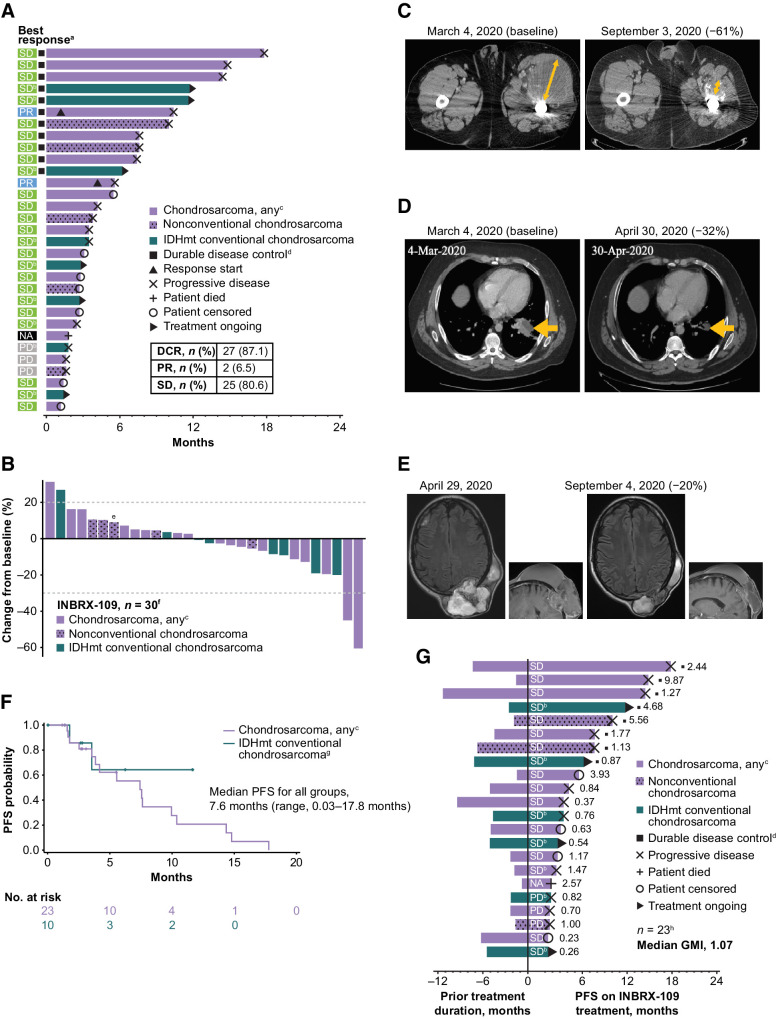
Figure 4.
Clinical efficacy of INBRX-109 in chondrosarcoma. A, Best tumor response and time receiving treatment up to the first event of progression or death. B, Best response up to data cutoff date. C and D, Representative baseline and posttreatment contrast-enhanced CT scans of patients who experienced a PR. Orange arrows show the diameter of target lesions. E, Representative baseline and posttreatment contrast-enhanced CT scans of a patient who experienced SD. All patients in C–E had grade 3, metastatic, conventional chondrosarcoma. F, PFS by Kaplan–Meier analysis. Median follow-up was 11.6 months. Crosses indicate censored data. G, Mean modified growth modulation index (modified GMI), as determined by the ratio of PFS with INBRX-109 to prior treatment duration. Best response to treatment with INBRX-109 is indicated within each bar, and the ratio of PFS on INBRX-109 to prior treatment duration is indicated at the right of each bar. Data cutoff: May 26, 2022. Abbreviations: GMI, growth modulation index; IDHmt, isocitrate dehydrogenase 1/2 mutant; NA, no postbaseline scan available; PD, progressive disease. aA total of 31 patients were included in the analysis. Four patients from cohort B6 were excluded for taking prohibited medications (n = 1), not having conventional chondrosarcoma (n = 1), or not having first scan data (n = 2). bPatient has a mutation in IDH1 (R132) or IDH2 (R172). cOne patient is from dose-escalation cohort A4 and received INBRX-109 at a dose of 10 mg/kg; all other patients are from dose-expansion cohort B4 (INBRX-109 3 mg/kg). Patients with nonconventional chondrosarcoma are indicated with a stippled pattern. dDurable disease control is SD, PR, or CR for >6 months. ePatient's first tumor assessment during treatment showed PD. A second scan post PD showed tumor shrinkage, and the best change from baseline up to data cutoff is shown; patient was not receiving a subsequent therapy at the time of the second scan. fA total of 30 patients were evaluable. Five patients were excluded for taking prohibited medications (n = 1; cohort B6), not having conventional chondrosarcoma (n = 1; cohort B6), not having first scan data (n = 2; cohort B6), or due to death (n = 1; cohort B4). gTwo patients were excluded due to taking prohibited medication (n = 1) or having dedifferentiated chondrosarcoma (n = 1). hOverall, 23 of 35 patients were included in this analysis. One patient had a long prior treatment duration of 84 months (cohort B6) and, although included in the analysis, is not included in the figure (GMI, 0.03). Patients were excluded for taking prohibited medications (n = 1; cohort B6), not having conventional chondrosarcoma (n = 1; cohort B6), or having a treatment duration that could not be estimated. (n = 10). Partial start and stop dates were ignored.