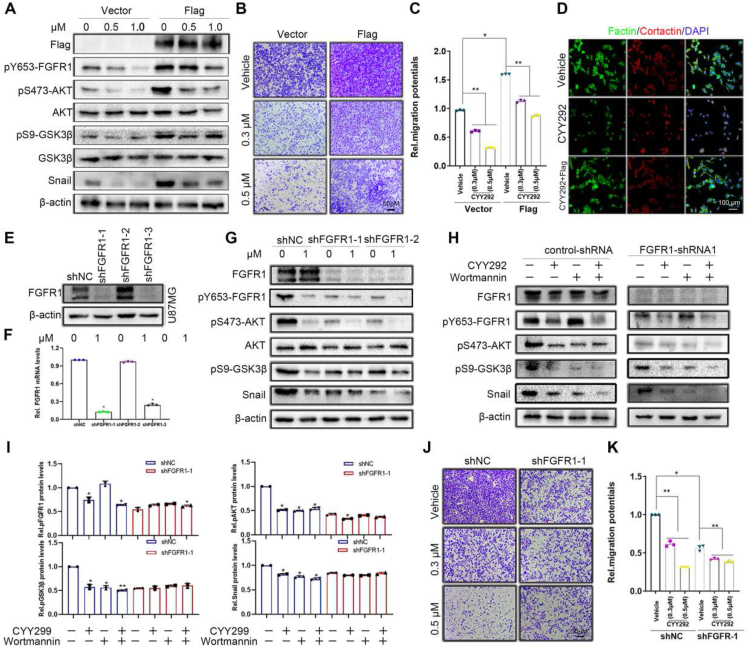
Figure 4.
CYY292 blocks EMT by inactivating the Akt/GSK3β/Snail pathway. (A) FGFR1 stably (3 × FLAG-FGFR1) expressing cells are sufficient to increase the phosphorylation of FGFR1, AKT, GSK3β, and Snail. U87MG were infected with a plasmid expressing FGFR1 or a control. Cells were treated with dose-dependent CYY292 for 24 h. (B) Boyden chamber migration assays of U87MG cells stably expressing vehicle or Flag-FGFR1. (C) Quantification of invaded cells shown in (B). n = 3 independent experiments. (D) Immunofluorescent staining of F-actin and cortactin in vehicle-treated, CYY292-treated, or Flag-CYY292-treated U87MG cells. Nuclear, DAPI (blue). (E) U87MG cells were stably transfected with negative control shRNA or shRNA targeting FGFR1. (F) mRNA expression of FGFR1 in FGFR1-knockdown U87MG normalized to control cells (n = 3 independent experiments). (G) Comparison of p-FGFR1/FGFR1, p-AKT/AKT, p-GSK3β/GSK3β, and Snail expressions in control and FGFR1-deleted U87MG cells treated with vehicle or CYY292 for 24 h. β-Actin was used as a loading control. (H) Comparison of p-FGFR1, p-AKT, p-GSK3β, and Snail expressions in control (left) and FGFR1-deleted (right) U87MG cells treated with vehicle or CYY292 for 24 h. Cells were pre-treated with either wortmannin (0.1 μM) for 2 h. All representative blots and images as shown are from three independent experiments. (I) Quantification of p-FGFR1, p-AKT, and p-GSK3β levels in control (left) and FGFR1-deleted (right) U87MG cells. All data are presented as means ± SD (n = 3 independent experiments). ∗P < 0.05. (J, K) Equal numbers of control and FGFR1-deleted U87MG cells pretreated with vehicle or CYY292 for 24 h were subjected to cell migration assays, and invaded cells were quantified (K). All data are presented as means ± SD (n = 3 independent experiments). ∗P < 0.05, ∗∗P < 0.01, #P > 0.05. Differences are tested using one-way analysis of variance (ANOVA) with Tukey's post hoc test.