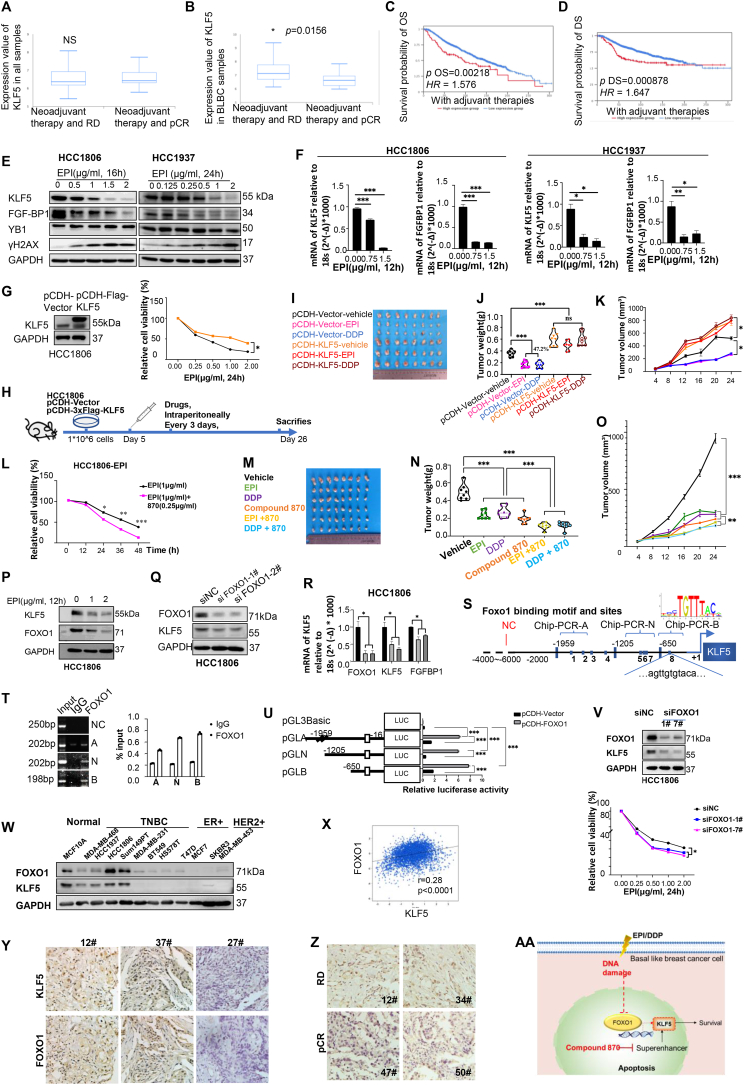
Figure 1.
DNA-damaging drugs down-regulate KLF5 transcription by inhibiting FOXO1. (A) KLF5 expression in samples from all kinds of patients with breast cancer presenting RD and pCR after adjuvant chemotherapy. No significant difference was observed (P = 0.602). (B) In patients with triple-negative breast cancer receiving neoadjuvant chemotherapy, the level of KLF5 is significantly higher in the RD group than that in the pCR group (P = 0.0156). BLBC, basal-like breast cancer. The overall survival (C) and disease-free survival (D) in patients with high KLF5 expression are significantly worse than those in patients with low KLF5 expression. OS, overall survival; DS, disease-free survival. OS: P = 0.00218, HR = 1.576; DS: P = 0.000878, HR = 1.647). (E, F) HCC1806 and HCC1937 cells were treated with EPI for 24 h, and the expression of KLF5 and downstream genes of FGF-BP1 were detected by western blotting and real-time PCR. (G) HCC1806 and HCC1937 cells overexpressing KLF5 were treated with DDP and EPI for 24 h, and cell viability was detected by the SRB assay. (H) KLF5-overexpressing HCC1806 cells and control cells were transplanted into BALB/c nude mice (female, 6–8 weeks old). n = 4 in each group. EPI or DDP was administered intraperitoneally to tumors at day 6, every 4 days for 20 days. The tumor size (I), tumor weight (J), and tumor volume (K) were measured for each group. (L) HCC1806 cells were treated with chemotherapeutic EPI in combination with drugs that down-regulate KLF5 (compound 870) for 24 h, and cell activity was detected using the SRB assay. (M–O) HCC1806 cells were transplanted into BALB/c nude mice, female, 6–8 weeks old, n = 4 in each group. The tumors were treated with EPI or DDP, or combined with compound 870 on day 5 after injection, intraperitoneally, every 4 days for 20 days. The tumor size (M), tumor weight (N), and tumor volume (O) were measured in each group. (P) HCC1806 cells were treated with EPI for 12 h, and the down-regulation of KLF5 and FOXO1 was detected by western blotting. (Q, R) siRNA knockdown of FOXO1 decreases protein and mRNA levels of KLF5 in HCC1806 cells. (S) JASPAR prediction of binding sites of FOXO1 on the KLF5 promoter at Homo sapiens chromosome 13, GRCh38.p13, NC_000013.11:73052976-73054975. (T) HCC1806 cells were subjected to ChIP-PCR. (U) HEK293T cells transfected with pGL3-KLF5-promoter A (−1500 bp), N (−1000 bp), and B (−700 bp) (−553∼−542) were subjected to the dual luciferase assay. (V) siRNAs-mediated FOXO1-down-regulated HCC1806 cells were treated with EPI for 24 h, and cell viability was detected using the SRB assay. (W) The expression of FOXO1 and KLF5 in different breast cancer cell lines was measured using western blotting. (X) The co-expression of KLF5 and FOXO1 was analyzed in the TCGA database. (Y) The expression of KLF5 and FOXO1 in TNBC samples. 12# and 37# are two representatives of positive samples, and 27# is a representative of negative sample. (Z) The FOXO1 expression in samples developing RD or pCR after neoadjuvant chemotherapies. (AA) The working model of FOXO1 and KLF5 regulation in breast cancer cells treated with DNA damage drugs. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. ChIP-PCR, chromatin-immunoprecipitation-PCR; DDP, cisplatin; EP, epirubicin; FOXO1, Forkhead box class O 1; KLF5, Krüppel-like factor 5; pCR, pathological complete response; RD, residual disease; SRB, sulforhodamine B; TCGA, The Cancer Genome Atlas; TNBC, triple-negative breast cancer.