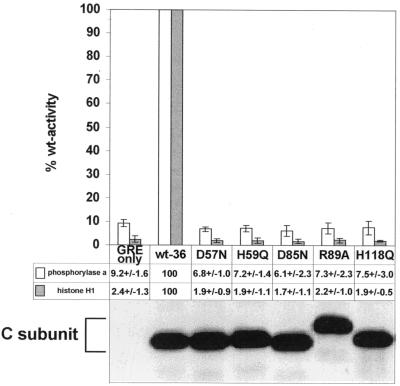
FIG. 2.
Analysis of the phosphatase activity in immunoprecipitates of epitope-tagged wild-type and mutant C subunits. Lysates of cell lines expressing the control vector (GREonly) or epitope-tagged wt (wt-36) or mutant C subunits were immunoprecipitated with 12CA5 antibody. After being washed, the immunoprecipitates were split into three parts and analyzed by SDS-PAGE and immunoblotting or tested for catalytic activity towards phosphorylase a and cdc2-phosphorylated histone H1 as substrates. The C subunit mutants are referred to by the mutated position preceded by the wt residue and followed by the introduced amino acid. The immunoblot below the graph shows that comparable amounts of immunoprecipitated wt and mutant C subunits were tested for catalytic activities. This anti-epitope tag antibody immunoblot of anti-epitope tag C subunit immunoprecipitates was generated from one of the four experiments used for the determination of the phosphatase activities and is representative of the other blots. Phosphatase activities of the vector control and mutant C subunit immunoprecipitates are presented below the graph as percentages of the activity obtained for immunoprecipitated wt C subunit. The mean values ± standard deviations of four independent experiments are depicted in column graphs. The data for H59Q and H118Q are from Ogris et al. (46). The values obtained for the vector control, GREonly, are most likely due to nonspecific binding of endogenous phosphatases from the cell lysates.