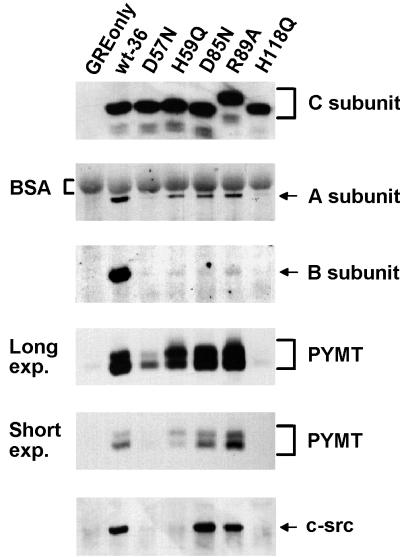
FIG. 3.
Complex formation of mutant PP2A C subunits with cellular and viral PP2A regulatory subunits and with pp60c-src. Lysates of cell lines expressing the control vector (GREonly) or epitope-tagged wt (wt-36) or mutant C subunits (see Fig. 2 for nomenclature) were immunoprecipitated with 12CA5 antibody and analyzed by SDS-PAGE and immunoblotting. The Western blot was sequentially probed with antibodies recognizing the epitope-tagged C subunit, A subunit, B subunit, polyomavirus MT (PYMT), and pp60c-src (c-src). Two different exposures (exp.) of the MT panel are shown. MT in complex with wt and inactive C subunits migrates as a doublet in SDS-PAGE, although the relative abundance of the two bands varies depending on the particular C subunit. The altered migration of the R89A mutant C subunit in SDS-PAGE was constantly seen and seems to be due to the substitution of the R, since several other amino acid changes at this position caused the same effect (data not shown). In the A subunit panel, background signal from bovine serum albumin (BSA), which was used for preblocking of the protein A-Sepharose beads, is indicated with brackets. The data shown are representative of results obtained from four independent experiments.