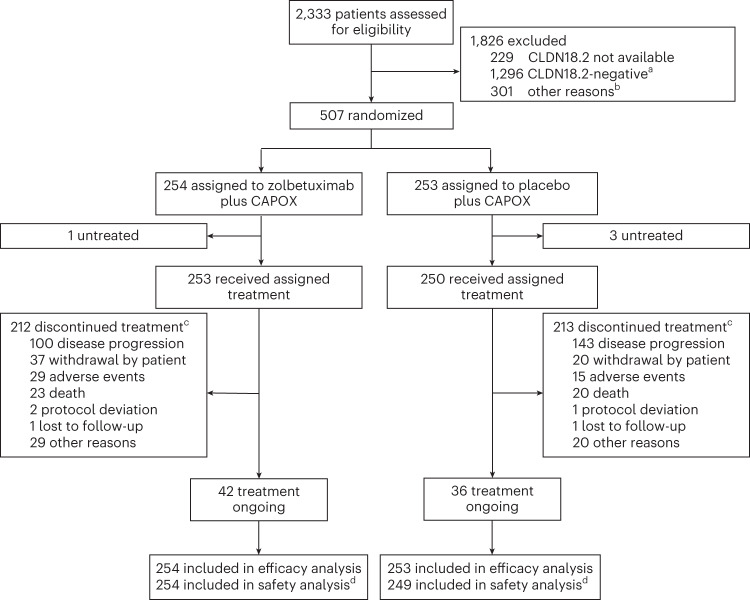
Fig. 1. CONSORT diagram of GLOW study.
a‘CLDN18.2-positive’ was defined as ≥75% of tumor cells with moderate-to-strong membranous CLDN18 staining as determined by central immunohistochemistry using the investigational VENTANA CLDN18 (43-14A) RxDx Assay. b‘Other’ represents patients whose tumors were CLDN18.2-positive but failed screening for other reasons, including laboratory findings, HER2 expression status, Eastern Cooperative Oncology Group (ECOG) performance status score, other exclusion criteria or withdrawal by patient. cIf patients discontinued from both zolbetuximab or placebo and CAPOX on the same day, all reasons for discontinuation were summarized; the sum of values for individual reasons for discontinuation is more than 212 for the zolbetuximab group and more than 213 for the placebo group. dOne patient randomized to the placebo plus CAPOX group received one dose of zolbetuximab as a protocol deviation and was moved to the zolbetuximab plus CAPOX group for the safety analysis set.