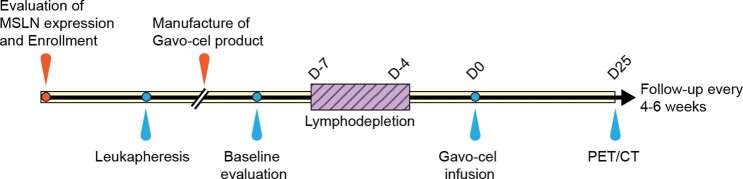
Extended Data Fig. 1. Schematic representation of the Gavo-cel study design.
Tumor biopsies were evaluated for mesothelin (MSLN) expression at enrolment, followed by leukapheresis and gavo-cel manufacture. The enrolled patients underwent lymphodepletion with fludarabine at 30 mg/m2/day, from days -7 to -4 and cyclophosphamide at 600 mg/m2/day from days -6 to -4. On day 0, they were infused with different doses of gavo-cel. Positron emission tomography (PET) and computerized tomography (CT) scans were performed at baseline, and at 1, 2, 3, 6 and 9, and 12 months after gavo-cel infusion.