Abstract
Granular corneal dystrophy type 2 (GCD2) is an autosomal dominant corneal stromal dystrophy that is caused by p.Arg124His mutation of transforming growth factor β induced (TGFBI) gene. It is characterized by well demarcated granular shaped opacities in central anterior stroma and as the disease progresses, extrusion of the deposits results in ocular pain due to corneal epithelial erosion. Also, diffuse corneal haze which appears late, causes decrease in visual acuity. The prevalence of GCD2 is high in East Asia including Korea. Homozygous patients show a severe phenotype from an early age, and the heterozygote phenotype varies among patients, depending on several types of compound heterozygous TGFBI mutations. In the initial stage, conservative treatments such as artificial tears, antibiotic eye drops, and bandage contact lenses are used to treat corneal erosion. Different surgical methods are used depending on the depth and extent of the stromal deposits. Phototherapeutic keratectomy removes anterior opacities and is advantageous in terms of its applicability and repeatability. For deeper lesions, deep anterior lamellar keratoplasty can be used as the endothelial layer is not always affected. Recurrence following these treatments are reported within a wide range of rates in different studies due to varying definition of recurrence and follow-up period. In patients who have undergone corneal laser vision-correction surgeries such as photorefractive keratectomy, LASEK, or LASIK including SMILE surgery, corneal opacity exacerbates rapidly with severe deterioration of visual acuity. Further investigations on new treatments of GCD2 are necessary.
Keywords: Corneal dystrophy Avellino type, Hereditary corneal dystrophies, Granular corneal dystrophy type 2
Introduction
Corneal dystrophies are rare hereditary corneal diseases that are usually bilateral, symmetric, slowly progressing, and are unrelated to systemic factors [1]. The disease is manifested in the form of the deposit accumulation in the various layers of the cornea. Previously, the classification of corneal dystrophy had been mainly based on the location of corneal opacity and the characteristics of the phenotype. Recently, the International Committee for Classification of Corneal Dystrophy (IC3D) developed a new classification system based on different layers of the cornea of which that is affected [2,3]. Corneal dystrophies are currently divided into epithelial and subepithelial dystrophies, epithelial-stromal transforming growth factor β induced (TGFBI) dystrophies, stromal dystrophies, and endothelial dystrophies.
Granular corneal dystrophy type 2 (GCD2) was first described by Bücklers in 1938, which, however, was considered as a mild variant of GCD1 for a long period of time [2]. GCD2 is inherited by autosomal dominant pattern with very high penetrance, showing granular deposits, linear lesions in deep stroma and superficial diffuse haze in advanced stage [2,4]. Because this corneal dystrophy showed different phenotype compared to GCD1, it was separated from GCD1 in 1992. As a pedigree of the dystrophy was established with a family originated from Avellino district of Italy, it was named Avellino corneal dystrophy at first [5]. Then, in 2008, IC3D recommended expressing this corneal dystrophy as GCD2 [3].
This review aims to outline key knowledges regarding genetics, epidemiology, clinical features, and management of GCD2.
Genetics, Epidemiology, and Pathophysiology
GCD2 is caused by mutations of TGFBI gene on chromosome 5q31 and is inherited by autosomal dominant fashion with high penetrance. One of the most common mutations is p.Arg124His mutation [6,7]. The other mutations inducing morphologic or histologic characteristics similar to that of GCD2 need further evaluation [8–11].
Even though GCD2 was once referred as Avellino corneal dystrophy, named after the Italian district as mentioned above, its prevalence is actually higher in East Asian countries such as Korea and Japan [12], being about 11.5 affected persons per 10,000 individuals in Korea [13]. GCD2 accounts for 72% to 91% of TGFBI-related corneal dystrophies in Korea and Japan, 67% in China, while it accounts for 36% in the United States and 3% in Poland [14,15].
TGFBI encodes for an extracellular matrix protein (TGF-BI protein [TGFBIp], also called keratoepithelin) involved in cell adhesion, migration, and proliferation [16]. Mutated TGFBIp aggregates and results in the formation of abnormal insoluble deposits within the corneal stroma. Recent research also showed that mutated TGFBIp itself affects the corneal fibroblast so that the function and characteristics of corneal fibroblasts would be changed in GCD2 [16,17].
Clinical Features
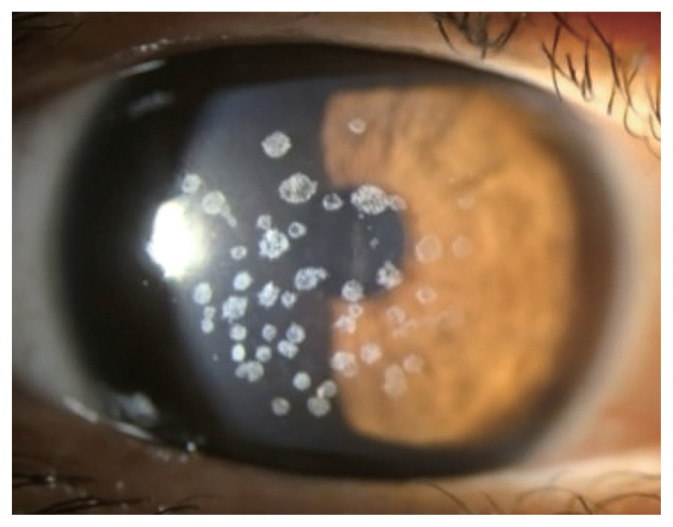
Small gray to white, well-circumscribed deposits appear in superficial stroma initially, and become larger into granular shapes (Fig. 1). As the disease progresses, the deposits become even larger and more pronounced. The center of the deposits can be dropped out, and extruded deposits cause corneal erosions, resulting in pain, photophobia, discomfort, and foreign body sensation [18]. However, recurrent corneal erosion are less frequent compared to GCD1 [12]. Center of the deposit forms a punched-out lesion leaving the lesion ring or discoid shaped with transparent centers. GCD2 also can show diffuse haze in anterior stroma with aging, reducing visual acuity.
Fig. 1.
Slit-lamp photography of granular corneal dystrophy type 2 heterozygote.
Shapes of corneal opacities differ in stromal layers, yet endothelium is spared. In anterior to mid stroma, it tends to be stellate and spiked, partially translucent in retroillumination while more linear and lattice like deposits are found in deeper stroma. Linear deposits are whiter and opaque, less refractile than that of lattice corneal dystrophy type 1.
Granular lesions are composed of hyaline, where linear lesions are composed of amyloid and linear deposits characteristically intersect. The granular opacities are shown as eosinophilic deposits using light microscopy under the Masson trichrome staining [19]. Upon transmission electron microscopy, rod-shaped electron dense deposits are found in anterior stroma. GCD2 heterozygous keratocytes are enlarged compared to normal corneal keratocytes, enabling visualization of intracellular organelle [20].
Clinical manifestations differ depending on homozygosity; homozygous GCD2 tends to be diagnosed earlier, be more severe and progresses more rapidly with significant visual impairment at early ages (Fig. 2). Homozygous patients can be diagnosed as early as at the age of 3 years, when white deposits on cornea becomes apparent [11]. Heterozygous patients are usually diagnosed as teenagers or young adults, although earliest can be at the age of 8 years [11]. Severity of heterozygote GCD2 can vary among the patients [9,10,21–30]. Several severe phenotypic forms were found to be caused by different types of compound heterozygous mutation [31].
Fig. 2.
Slit-lamp photography of granular corneal dystrophy type 2 homozygote.
Opacities are usually found around the center of the cornea, sparing periphery near the limbus. A study done by Lee et al. [32] showed that corneal neovascularization affects this feature. For instance, a GCD2 patient undergoing phthisis with corneal neovascularization had no deposits on the corneal periphery. In a GCD2 heterozygous patient with pterygium, opacities are located further away from limbus on the side of cornea where pterygium is present due to neovascularization, compared to the opposite side of the cornea without pterygium, where opacities are present closer to the limbus. Also, the opacities near pterygium tend to be less distinctive, implying resorption of the deposits. These findings show that characteristic of corneal vascular supply may be related to clearing of GCD2 deposits. Another supporting case is that clear incision near limbus during cataract surgery does not aggravate the disease whereas refractive surgeries such as laser in situ keratomileusis (LASIK) and laser epithelial keratomileusis (LASEK) performed at the central part of cornea, which is located far from the vascularized limbus, usually result in exacerbation of GCD2 [33].
Homozygous GCD2, compared to heterozygotes, shows more aggressive presentation and progression in terms of number of opacities, growth in size and fusion of the deposits. Moreover, patient’s vision is impaired more rapidly that he or she requires surgical treatments such as phototherapeutic keratectomy (PTK) or corneal transplantation even at childhood and recurrence is more frequent and faster [11]. Range of peripheral cornea that is clear of opacities decreases with age. In homozygous GCD2, the confluent opacities appear early but deep linear deposits do not form unlike heterozygous GCD2. Homozygous GCD2 can show two distinct phenotypes, type I opacity shows spot like gray-to-whitish deposits [11,34,35] and type II is characterized by grayish reticular shaped opacities with intervening translucent spaces resulting in better best corrected vision than type I opacity [36,37].
Several studies examined exacerbation or recurrence of GCD2 following different refractive surgeries such as LASIK, LASEK, and photorefractive keratectomy (PRK) [38–43]. After an uncomplicated LASIK surgery, opacities tend to get worse, forming multiple small granular deposits in between the flap and the stromal bed (Fig. 3), whereas fine deposits form at superficial stroma following LASEK and PRK. Exacerbation is generally more severe and final visual acuity is worse after LASIK compared to post-PRK status [42,43]. There is also a recent report where GCD2 aggravated after small incision lenticule extraction (SMILE) procedure [44].
Fig. 3.
Slit-lamp photography of granular corneal dystrophy type 2 cornea exacerbated after laser in situ keratomileusis (LASIK).
Management
Conservative managements, such as artificial tears or therapeutic contact lenses are utilized when GCD2 causes recurrent corneal erosions. Further surgical managements are considered when patients experience decrease in visual acuity, contrast sensitivity and therefore deterioration of functional vision. Choice of surgical method depends on depth and extent of the deposits and final treatment goal is to remove the opacities and postpone keratoplasty as late as possible.
PTK aims to remove opacities located in anterior stroma (Fig. 4A, 4B). It is easily applicable and does not impose risk of graft rejection. It delays timing of keratoplasty such as deep anterior lamellar keratoplasty (DALK) or penetrating keratoplasty (PKP) and can be done repeatedly despite recurrence and postkeratoplasty status [45]. Along with preoperative slit-lamp examination, Fourier domain optical coherence tomography (FD-OCT) can be utilized in order to visualize the depth of the corneal deposits, enable the surgeon to plan and minimize the ablation depth of PTK for removal of the deposits [46,47].
Fig. 4.
Phototherapeutic keratectomy in granular corneal dystrophy type 2 (GCD2). (A) Preoperative cornea of GCD2 patient. (B) Slitlamp photography of the GCD2 patient at 1 month after phototherapeutic keratectomy.
Recurrence rate reported in various studies range from 0% to 100% due to different definitions of recurrence and range of follow-up durations [48–54]. Deposits relapsed faster in homozygous patients at 18 months after first PTK treatment, and at 3 months following second or third treatment [11]. In a study done by Inoue et al. [55], GCD2 recurred after 9.5 months in four homozygous patients’ eyes, compared to 38.4 months in seven heterozygous GCD2 eyes. The use of mitomycin C (MMC) during PTK has been tried in some clinics. A 3-year follow-up after surface ablation using MMC showed that GCD2 still aggravated afterwards [56]. Use of MMC is not recommended as MMC would induce apoptosis of keratocyte which would do the work of reabsorption and degradation of the TGF-BIp located in the stroma [57,58].
PTK can be also done prior to or after different ocular surgeries. For example, PTK can be applied to cornea under progression after LASIK, with better effect when the LASIK flap is removed [54]. When a patient requires both cataract surgery and PTK, PTK should be done prior to, then different formulas for calculation of intraocular lens power after PTK can be used [59–61].
Keratoplasty such as anterior lamellar keratoplasty (ALKP), DALK, and PKP can be considered for deep stromal deposits and when PTK is no longer repeatable due to excessive corneal thinning, astigmatism, or scarring. Since corneal endothelium is spared in GCD2, ALKP and DALK is preferred over PKP. Recurrence rates are reported ranging from 0% to 71% depending on length of follow-up period, and some studies also did not mention subtypes of GCD [62–65].
Since corneal epithelial cells are suggested to be the origin of TGFBIp resulting in corneal deposits of GCDs in previous studies, limbal cell transplantation can be used. However, concerning the rejection of the transplanted limbal cells, it is not yet practically used [66–69]. Another possible treatment method reported for the removal of corneal deposits is corneal electrolysis. In a study done by Mashima et al. [70], corneal electrolysis was applied to two homozygous eyes that recurred following keratoplasty, where it recurred with fine granular deposits after three years. Long-term clinical outcome is not yet studied.
Future Perspectives
Further studies are needed to evaluate long-term clinical outcomes of different treatment modalities. Also, different management approaches other than surgical or interventional methods can also be considered for treatment of GCD2. For instance, studies have found that lithium chloride decreases TGFBI protein production [71] and combined treatment of melatonin and rapamycin inhibits TGF-BI protein expression while increasing degradation of the molecule [17,72,73]. In addition to pharmacological agents, gene therapy such as small interfering RNA (siRNA), short hairpin siRNA may be used to silence mutant TGFBI expression [74,75]. Genome editing technique such as CRISPR/Cas9 may be used to target genes site specifically. However, one of the problems of using gene therapy technique is the unwanted off-target effects, which may affect or silence the opposite normal allele or other genes [31,76]. Developing safe delivery methods free from unwanted off-target effects would be essential for specifically targeted gene therapy for GCD2.
Conclusion
GCD2 is a stromal corneal dystrophy inherited by an autosomal dominant manner. The dystrophy is characterized by well-circumscribed granular opacities, deep linear lesions and diffuse corneal haze resulting in decrease in visual acuity. Circumscribed superficial granular opacities may sometimes become punched-out lesions causing pain due to corneal erosions. Severity and pace of progression vary depending on the genotype, homozygotes being the most severe. Current managements include conservative treatment such as artificial tears and therapeutic contacts lenses for corneal erosions, and surgical interventions depending on the depth of the stromal deposits: PTK for anterior opacities and keratoplasty (DALK) for deeply located deposits. Further studies can be considered to develop different treatment modalities such as pharmacological agents or genetic therapy, providing wider range of options for GCD2 patients’ life-long management.
Acknowledgements
None.
Footnotes
Conflicts of Interest: Eung Kweon Kim is a member of the medical advisory board of Avellino Lab USA Inc (Menlo Park, CA, USA). However, he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Funding: This work was supported by the Basic Science Research Program (No. NRF-2021R1I1A1A01047951) of the National Research Foundation (NRF) funded by the Minsitry of Science and ICT of Korea and the Korean Fund for Regenerative Medicine (KFRM) grant (No. KFRM 22C0615L1) funded by the Ministry of Health and Welfare of Korea. The funding organization had no role in the design or conduct of this study.
References
- 1.Aggarwal S, Peck T, Golen J, Karcioglu ZA. Macular corneal dystrophy: a review. Surv Ophthalmol. 2018;63:609–17. doi: 10.1016/j.survophthal.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Weiss JS, Moller HU, Aldave AJ, et al. IC3D classification of corneal dystrophies: edition 2. Cornea. 2015;34:117–59. doi: 10.1097/ICO.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 3.Weiss JS, Moller HU, Lisch W, et al. The IC3D classification of the corneal dystrophies. Cornea. 2008;27(Suppl 2):S1–83. doi: 10.1097/ICO.0b013e31817780fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han KE, Kim TI, Chung WS, et al. Clinical findings and treatments of granular corneal dystrophy type 2 (avellino corneal dystrophy): a review of the literature. Eye Contact Lens. 2010;36:296–9. doi: 10.1097/ICL.0b013e3181ef0da0. [DOI] [PubMed] [Google Scholar]
- 5.Holland EJ, Daya SM, Stone EM, et al. Avellino corneal dystrophy. Clinical manifestations and natural history. Ophthalmology. 1992;99:1564–8. doi: 10.1016/s0161-6420(92)31766-x. [DOI] [PubMed] [Google Scholar]
- 6.Munier FL, Korvatska E, Djemai A, et al. Kerato-epithelin mutations in four 5q31-linked corneal dystrophies. Nat Genet. 1997;15:247–51. doi: 10.1038/ng0397-247. [DOI] [PubMed] [Google Scholar]
- 7.Korvatska E, Munier FL, Djemai A, et al. Mutation hot spots in 5q31-linked corneal dystrophies. Am J Hum Genet. 1998;62:320–4. doi: 10.1086/301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Streeten BW, Qi Y, Klintworth GK, et al. Immunolocalization of beta ig-h3 protein in 5q31-linked corneal dystrophies and normal corneas. Arch Ophthalmol. 1999;117:67–75. doi: 10.1001/archopht.117.1.67. [DOI] [PubMed] [Google Scholar]
- 9.Dighiero P, Drunat S, D’Hermies F, et al. A novel variant of granular corneal dystrophy caused by association of 2 mutations in the TGFBI gene-R124L and DeltaT125-DeltaE126. Arch Ophthalmol. 2000;118:814–8. doi: 10.1001/archopht.118.6.814. [DOI] [PubMed] [Google Scholar]
- 10.Sakimoto T, Kanno H, Shoji J, et al. A novel nonsense mutation with a compound heterozygous mutation in TGFBI gene in lattice corneal dystrophy type I. Jpn J Ophthalmol. 2003;47:13–7. doi: 10.1016/s0021-5155(02)00644-5. [DOI] [PubMed] [Google Scholar]
- 11.Moon JW, Kim SW, Kim TI, et al. Homozygous granular corneal dystrophy type II (Avellino corneal dystrophy): natural history and progression after treatment. Cornea. 2007;26:1095–100. doi: 10.1097/ICO.0b013e3181484013. [DOI] [PubMed] [Google Scholar]
- 12.Lisch W, Weiss JS. Clinical and genetic update of corneal dystrophies. Exp Eye Res. 2019;186:107715. doi: 10.1016/j.exer.2019.107715. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Cristol SM, Kim WC, et al. Prevalence of granular corneal dystrophy type 2 (Avellino corneal dystrophy) in the Korean population. Ophthalmic Epidemiol. 2010;17:160–5. doi: 10.3109/09286581003624939. [DOI] [PubMed] [Google Scholar]
- 14.Afshari NA, Mullally JE, Afshari MA, et al. Survey of patients with granular, lattice, avellino, and Reis-Bücklers corneal dystrophies for mutations in the BIGH3 and gelsolin genes. Arch Ophthalmol. 2001;119:16–22. [PubMed] [Google Scholar]
- 15.Nowinska AK, Wylegala E, Janiszewska DA, et al. Genotype-phenotype correlation of TGFBI corneal dystrophies in Polish patients. Mol Vis. 2011;17:2333–42. [PMC free article] [PubMed] [Google Scholar]
- 16.Han KE, Choi SI, Kim TI, et al. Pathogenesis and treatments of TGFBI corneal dystrophies. Prog Retin Eye Res. 2016;50:67–88. doi: 10.1016/j.preteyeres.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Choi SI, Kim BY, Dadakhujaev S, et al. Impaired autophagy and delayed autophagic clearance of transforming growth factor β-induced protein (TGFBI) in granular corneal dystrophy type 2. Autophagy. 2012;8:1782–97. doi: 10.4161/auto.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han KE, Chung WS, Kim T, et al. Changes of clinical manifestation of granular corneal deposits because of recurrent corneal erosion in granular corneal dystrophy types 1 and 2. Cornea. 2013;32:e113–20. doi: 10.1097/ICO.0b013e3182700620. [DOI] [PubMed] [Google Scholar]
- 19.Folberg R, Alfonso E, Croxatto JO, et al. Clinically atypical granular corneal dystrophy with pathologic features of lattice-like amyloid deposits. A study of these families. Ophthalmology. 1988;95:46–51. doi: 10.1016/s0161-6420(88)33226-4. [DOI] [PubMed] [Google Scholar]
- 20.Kim TI, Kim H, Lee DJ, et al. Altered mitochondrial function in type 2 granular corneal dystrophy. Am J Pathol. 2011;179:684–92. doi: 10.1016/j.ajpath.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aldave AJ, Gutmark JG, Yellore VS, et al. Lattice corneal dystrophy associated with the Ala546Asp and Pro551Gln missense changes in the TGFBI gene. Am J Ophthalmol. 2004;138:772–81. doi: 10.1016/j.ajo.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Klintworth GK, Bao W, Afshari NA. Two mutations in the TGFBI (BIGH3) gene associated with lattice corneal dystrophy in an extensively studied family. Invest Ophthalmol Vis Sci. 2004;45:1382–8. doi: 10.1167/iovs.03-1228. [DOI] [PubMed] [Google Scholar]
- 23.Frising M, Wildhardt G, Frisch L, Pitz S. Recurrent granular dystrophy of the cornea: an unusual case. Cornea. 2006;25:614–7. doi: 10.1097/01.ico.0000214202.84081.a5. [DOI] [PubMed] [Google Scholar]
- 24.Yamada N, Kawamoto K, Morishige N, et al. Double mutation (R124H, N544S) of TGFBI in two sisters with combined expression of Avellino and lattice corneal dystrophies. Mol Vis. 2009;15:974–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong X, Chen S, Huang W, et al. Novel and known mutations of TGFBI, their genotype-phenotype correlation and structural modeling in 3 Chinese families with lattice corneal dystrophy. Mol Vis. 2010;16:224–30. [PMC free article] [PubMed] [Google Scholar]
- 26.Niel-Butschi F, Kantelip B, Iwaszkiewicz J, et al. Genotype-phenotype correlations of TGFBI p.Leu509Pro, p. Leu509Arg, p.Val613Gly, and the allelic association of p.Met502Val-p.Arg555Gln mutations. Mol Vis. 2011;17:1192–202. [PMC free article] [PubMed] [Google Scholar]
- 27.Yam GH, Wang K, Jhanji V, et al. In vitro amyloid aggregate forming ability of TGFBI mutants that cause corneal dystrophies. Invest Ophthalmol Vis Sci. 2012;53:5890–8. doi: 10.1167/iovs.11-9068. [DOI] [PubMed] [Google Scholar]
- 28.Song JS, Lim DH, Chung ES, et al. Mutation analysis of the TGFBI gene in consecutive Korean patients with corneal dystrophies. Ann Lab Med. 2015;35:336–40. doi: 10.3343/alm.2015.35.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chae H, Kim M, Kim Y, et al. Mutational spectrum of Korean patients with corneal dystrophy. Clin Genet. 2016;89:678–89. doi: 10.1111/cge.12726. [DOI] [PubMed] [Google Scholar]
- 30.Ann LB, Abbouda A, Frausto RF, et al. Variant lattice corneal dystrophy associated with compound heterozygous mutations in the TGFBI gene. Br J Ophthalmol. 2017;101:509–13. doi: 10.1136/bjophthalmol-2015-307602. [DOI] [PubMed] [Google Scholar]
- 31.Jun I, Ji YW, Choi SI, et al. Compound heterozygous mutations in TGFBI cause a severe phenotype of granular corneal dystrophy type 2. Sci Rep. 2021;11:6986. doi: 10.1038/s41598-021-86414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JH, Chung SH, Stulting RD, et al. Effects of corneal neovascularization on the manifestations of Avellino corneal dystrophy (granular corneal dystrophy type II) Cornea. 2006;25:914–8. doi: 10.1097/01.ico.0000224645.89342.55. [DOI] [PubMed] [Google Scholar]
- 33.Roh MI, Chung SH, Stulting RD, et al. Preserved peripheral corneal clarity after surgical trauma in patients with Avellino corneal dystrophy. Cornea. 2006;25:497–8. doi: 10.1097/01.ico.0000214236.59356.43. [DOI] [PubMed] [Google Scholar]
- 34.Mashima Y, Konishi M, Nakamura Y, et al. Severe form of juvenile corneal stromal dystrophy with homozygous R124H mutation in the keratoepithelin gene in five Japanese patients. Br J Ophthalmol. 1998;82:1280–4. doi: 10.1136/bjo.82.11.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada M, Yamamoto S, Inoue Y, et al. Severe corneal dystrophy phenotype caused by homozygous R124H keratoepithelin mutations. Invest Ophthalmol Vis Sci. 1998;39:1947–53. [PubMed] [Google Scholar]
- 36.Watanabe H, Hashida Y, Tsujikawa K, et al. Two patterns of opacity in corneal dystrophy caused by the homozygous BIG-H3 R124H mutation. Am J Ophthalmol. 2001;132:211–6. doi: 10.1016/s0002-9394(01)00962-x. [DOI] [PubMed] [Google Scholar]
- 37.Tsujikawa K, Tsujikawa M, Watanabe H, et al. Allelic homogeneity in Avellino corneal dystrophy due to a founder effect. J Hum Genet. 2007;52:92–7. doi: 10.1007/s10038-006-0083-4. [DOI] [PubMed] [Google Scholar]
- 38.Jun RM, Tchah H, Kim TI, et al. Avellino corneal dystrophy after LASIK. Ophthalmology. 2004;111:463–8. doi: 10.1016/j.ophtha.2003.06.026. [DOI] [PubMed] [Google Scholar]
- 39.Banning CS, Kim WC, Randleman JB, et al. Exacerbation of Avellino corneal dystrophy after LASIK in North America. Cornea. 2006;25:482–4. doi: 10.1097/01.ico.0000195949.93695.37. [DOI] [PubMed] [Google Scholar]
- 40.Roh MI, Grossniklaus HE, Chung SH, et al. Avellino corneal dystrophy exacerbated after LASIK: scanning electron microscopic findings. Cornea. 2006;25:306–11. doi: 10.1097/01.ico.0000183536.07275.9a. [DOI] [PubMed] [Google Scholar]
- 41.Aldave AJ, Sonmez B, Forstot SL, et al. A clinical and histopathologic examination of accelerated TGFBIp deposition after LASIK in combined granular-lattice corneal dystrophy. Am J Ophthalmol. 2007;143:416–9. doi: 10.1016/j.ajo.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 42.Kim TI, Kim T, Kim SW, Kim EK. Comparison of corneal deposits after LASIK and PRK in eyes with granular corneal dystrophy type II. J Refract Surg. 2008;24:392–5. doi: 10.3928/1081597X-20080401-13. [DOI] [PubMed] [Google Scholar]
- 43.Lee JH, Stulting RD, Lee DH, et al. Exacerbation of granular corneal dystrophy type II (Avellino corneal dystrophy) after LASEK. J Refract Surg. 2008;24:39–45. doi: 10.3928/1081597X-20080101-06. [DOI] [PubMed] [Google Scholar]
- 44.Kwak JJ, Yoon SH, Seo KY, et al. Exacerbation of granular corneal dystrophy type 2 after small incision lenticule extraction. Cornea. 2021;40:519–24. doi: 10.1097/ICO.0000000000002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maclean H, Robinson LP, Wechsler AW, Goh A. Excimer phototherapeutic keratectomy for recurrent granular dystrophy. Aust N Z J Ophthalmol. 1996;24:127–30. doi: 10.1111/j.1442-9071.1996.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 46.Kim TI, Hong JP, Ha BJ, et al. Determination of treatment strategies for granular corneal dystrophy type 2 using Fourier-domain optical coherence tomography. Br J Ophthalmol. 2010;94:341–5. doi: 10.1136/bjo.2009.164525. [DOI] [PubMed] [Google Scholar]
- 47.Jung SH, Han KE, Stulting RD, et al. Phototherapeutic keratectomy in diffuse stromal haze in granular corneal dystrophy type 2. Cornea. 2013;32:296–300. doi: 10.1097/ICO.0b013e31824a2288. [DOI] [PubMed] [Google Scholar]
- 48.Starr M, Donnenfeld E, Newton M, et al. Excimer laser phototherapeutic keratectomy. Cornea. 1996;15:557–65. [PubMed] [Google Scholar]
- 49.Orndahl MJ, Fagerholm PP. Treatment of corneal dystrophies with phototherapeutic keratectomy. J Refract Surg. 1998;14:129–35. doi: 10.3928/1081-597X-19980301-11. [DOI] [PubMed] [Google Scholar]
- 50.Dinh R, Rapuano CJ, Cohen EJ, Laibson PR. Recurrence of corneal dystrophy after excimer laser phototherapeutic keratectomy. Ophthalmology. 1999;106:1490–7. doi: 10.1016/S0161-6420(99)90441-4. [DOI] [PubMed] [Google Scholar]
- 51.Dogru M, Katakami C, Nishida T, Yamanaka A. Alteration of the ocular surface with recurrence of granular/avellino corneal dystrophy after phototherapeutic keratectomy: report of five cases and literature review. Ophthalmology. 2001;108:810–7. doi: 10.1016/s0161-6420(00)00657-6. [DOI] [PubMed] [Google Scholar]
- 52.Chen M, Xie L. Features of recurrence after excimer laser phototherapeutic keratectomy for anterior corneal pathologies in North China. Ophthalmology. 2013;120:1179–85. doi: 10.1016/j.ophtha.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Reddy JC, Rapuano CJ, Nagra PK, Hammersmith KM. Excimer laser phototherapeutic keratectomy in eyes with corneal stromal dystrophies with and without a corneal graft. Am J Ophthalmol. 2013;155:1111–8. doi: 10.1016/j.ajo.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 54.Jun I, Jung JW, Choi YJ, et al. Long-term clinical outcomes of phototherapeutic keratectomy in corneas with granular corneal dystrophy type 2 exacerbated after LASIK. J Refract Surg. 2018;34:132–9. doi: 10.3928/1081597X-20171220-01. [DOI] [PubMed] [Google Scholar]
- 55.Inoue T, Watanabe H, Yamamoto S, et al. Recurrence of corneal dystrophy resulting from an R124H Big-h3 mutation after phototherapeutic keratectomy. Cornea. 2002;21:570–3. doi: 10.1097/00003226-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Ha BJ, Kim TI, Choi SI, et al. Mitomycin C does not inhibit exacerbation of granular corneal dystrophy type II induced by refractive surface ablation. Cornea. 2010;29:490–6. doi: 10.1097/ICO.0b013e3181c3258a. [DOI] [PubMed] [Google Scholar]
- 57.Kim TI, Choi SI, Lee HK, et al. Mitomycin C induces apoptosis in cultured corneal fibroblasts derived from type II granular corneal dystrophy corneas. Mol Vis. 2008;14:1222–8. [PMC free article] [PubMed] [Google Scholar]
- 58.Choi SI, Maeng YS, Kim TI, et al. Lysosomal trafficking of TGFBIp via caveolae-mediated endocytosis. PLoS One. 2015;10:e0119561. doi: 10.1371/journal.pone.0119561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung SH, Han KE, Sgrignoli B, et al. Intraocular lens power calculations for cataract surgery after phototherapeutic keratectomy in granular corneal dystrophy type 2. J Refract Surg. 2012;28:714–24. doi: 10.3928/1081597X-20120921-07. [DOI] [PubMed] [Google Scholar]
- 60.Yagi-Yaguchi Y, Negishi K, Saiki M, et al. Comparison of the accuracy of newer intraocular lens power calculation methods in eyes that underwent previous phototherapeutic keratectomy. J Refract Surg. 2019;35:310–6. doi: 10.3928/1081597X-20190410-01. [DOI] [PubMed] [Google Scholar]
- 61.Yoneyama R, Kamiya K, Iijima K, et al. Predictability of intraocular lens power calculation in eyes after phototherapeutic keratectomy. Jpn J Ophthalmol. 2020;64:62–7. doi: 10.1007/s10384-019-00699-y. [DOI] [PubMed] [Google Scholar]
- 62.Ellies P, Renard G, Valleix S, et al. Clinical outcome of eight BIGH3-linked corneal dystrophies. Ophthalmology. 2002;109:793–7. doi: 10.1016/s0161-6420(01)01025-9. [DOI] [PubMed] [Google Scholar]
- 63.Park KA, Ki CS, Chung ES, Chung TY. Deep anterior lamellar keratoplasty in Korean patients with Avellino dystrophy. Cornea. 2007;26:1132–5. doi: 10.1097/ICO.0b013e318123765b. [DOI] [PubMed] [Google Scholar]
- 64.Salouti R, Hosseini H, Eghtedari M, Khalili MR. Deep anterior lamellar keratoplasty with melles technique for granular corneal dystrophy. Cornea. 2009;28:140–3. doi: 10.1097/ICO.0b013e3181861cdd. [DOI] [PubMed] [Google Scholar]
- 65.Unal M, Arslan OS, Atalay E, et al. Deep anterior lamellar keratoplasty for the treatment of stromal corneal dystrophies. Cornea. 2013;32:301–5. doi: 10.1097/ICO.0b013e31825718ca. [DOI] [PubMed] [Google Scholar]
- 66.Sundmacher R, Spelsberg H, Reinhard T. Homologous penetrating central limbokeratoplasty in granular and lattice corneal dystrophy. Cornea. 1999;18:664–70. doi: 10.1097/00003226-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 67.Dunaief JL, Ng EW, Goldberg MF. Corneal dystrophies of epithelial genesis: the possible therapeutic use of limbal stem cell transplantation. Arch Ophthalmol. 2001;119:120–2. [PubMed] [Google Scholar]
- 68.Spelsberg H, Reinhard T, Henke L, et al. Penetrating limbo-keratoplasty for granular and lattice corneal dystrophy: survival of donor limbal stem cells and intermediate-term clinical results. Ophthalmology. 2004;111:1528–33. doi: 10.1016/j.ophtha.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 69.Lang SJ, Eberwein P, Reinshagen H, et al. Simultaneous transplantation of limbal stem cells may reduce recurrences of granular dystrophy after corneal transplantation: 2 long-term case reports. Medicine (Baltimore) 2015;94:e789. doi: 10.1097/MD.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mashima Y, Kawai M, Yamada M. Corneal electrolysis for recurrence of corneal stormal dystrophy after keratoplasty. Br J Ophthalmol. 2002;86:273–5. doi: 10.1136/bjo.86.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choi SI, Kim BY, Dadakhujaev S, et al. Inhibition of TGF-BIp expression by lithium: implications for TGFBI-linked corneal dystrophy therapy. Invest Ophthalmol Vis Sci. 2011;52:3293–300. doi: 10.1167/iovs.10-6405. [DOI] [PubMed] [Google Scholar]
- 72.Choi SI, Dadakhujaev S, Ryu H, et al. Melatonin protects against oxidative stress in granular corneal dystrophy type 2 corneal fibroblasts by mechanisms that involve membrane melatonin receptors. J Pineal Res. 2011;51:94–103. doi: 10.1111/j.1600-079X.2011.00866.x. [DOI] [PubMed] [Google Scholar]
- 73.Choi SI, Kim KS, Oh JY, et al. Melatonin induces autophagy via an mTOR-dependent pathway and enhances clearance of mutant-TGFBIp. J Pineal Res. 2013;54:361–72. doi: 10.1111/jpi.12039. [DOI] [PubMed] [Google Scholar]
- 74.Yuan C, Zins EJ, Clark AF, Huang AJ. Suppression of keratoepithelin and myocilin by small interfering RNAs (siRNA) in vitro. Mol Vis. 2007;13:2083–95. [PubMed] [Google Scholar]
- 75.Courtney DG, Atkinson SD, Moore JE, et al. Development of allele-specific gene-silencing siRNAs for TGFBI Arg-124Cys in lattice corneal dystrophy type I. Invest Ophthalmol Vis Sci. 2014;55:977–85. doi: 10.1167/iovs.13-13279. [DOI] [PubMed] [Google Scholar]
- 76.Kim EK, Kim S, Maeng YS. Generation of TGFBI knockout ABCG2+/ABCB5+ double-positive limbal epithelial stem cells by CRISPR/Cas9-mediated genome editing. PLoS One. 2019;14:e0211864. doi: 10.1371/journal.pone.0211864. [DOI] [PMC free article] [PubMed] [Google Scholar]